Marine fish eggs and larvae from the east coast of South Africa
Allan Connell
Durban
South Africa
Originally posted May 2007; updated October 2012.
Abstract. Twentyfive years of records are summarised, in 226 separate species data sheets, of eggs and early larvae of fishes spawning pelagic eggs on the inshore shelf, within 5km of the coast, along a short section of the KwaZulu-Natal coastline, about 50km south of Durban. Annual spawning period, and egg abundance trends over the 25 years, are provided, as well as egg and larval descriptions, showing the ephemeral pigment patterns of many species' early larvae, in digital image colour. By collecting both offshore (5km) and inshore (0.5km) samples, comparing percentage representation of each species within these two sample sets, and using two indicator species, the kob Argyrosomus japonicus and the geelbek Atractoscion aequidens, both with well-defined spawning grounds, a reasonable assessment of location of spawning was obtained for all the common eggs in the study area. Roughly 65 species were reared on a simple food-chain for identification purposes, but in the latter stages of the study, larval identification was by DNA barcoding using the cytochrome C1 gene. The study is ongoing, both to increase the annual trend graphics for each species, and to gather barcodes of currently unidentified eggs and larvae, so that they will be identified when adult material has been sequenced, in support of which, over 900 species of local marine fishes have been barcoded. Introductory Notes describe the geography and oceanography of the study area, with particular emphasis on how they affect spawning migrations of species into the study area, and movement of early juveniles to their preferred nursery areas.
This study resulted from concern regarding the effects of effluent discharges into the marine environment, on the early life stages of marine fishes with pelagic eggs. In 1985, a major effluent pipeline was about to begin discharging industrial effluent over a shallow continental shelf area, offshore from the newly developed Richards Bay harbour, on the east coast of South Africa, in an area of widened shelf waters which was regarded as biologically productive in the local context (Figures 1 & 2). In an effort to assess whether the area was an important spawning ground for coastal marine fishes, a series of surface plankton samples was collected, over several years, to assess the diversity of fish species spawning in the area, and the intensity and seasonality of spawning. These samples were preserved with formalin, aboard ship, in order that they could be returned to the laboratory for further study.
 |
| Figure 1: Overview of the study area, on the east coast of South Africa |
The samples soon highlighted the difficulty of trying
to identify fish eggs fixed in formalin, without the benefit of being able to
hatch the eggs, and examine the pigment patterns and other diagnostic features
of the developing larva. Thus, while the Richards Bay study continued to
examine formalin-fixed samples, a 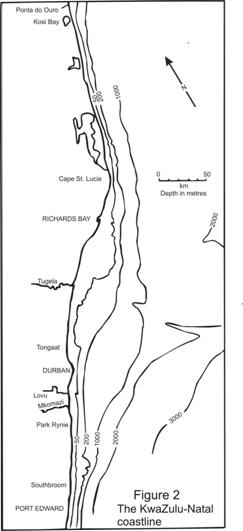 second study was started, closer to home.
This entailed collecting live plankton samples, using a surface-towed plankton
net, which enabled me to return the samples, alive, in 25 litre buckets of
seawater, to the laboratory for separation, cataloguing, and hatching of the
eggs. This latter study began in 1986, and careful cataloguing of eggs began in
January 1987.
second study was started, closer to home.
This entailed collecting live plankton samples, using a surface-towed plankton
net, which enabled me to return the samples, alive, in 25 litre buckets of
seawater, to the laboratory for separation, cataloguing, and hatching of the
eggs. This latter study began in 1986, and careful cataloguing of eggs began in
January 1987.
During the course of the study, it became apparent that certain important species were excluded, as they were attracted to estuarine systems. Thus, over the period May 1990 to December 1994, a series of samples was collected from the entrance to Durban harbour. Some of these eggs were rarely or never encountered in the coastal samples, adding insight into the importance of Durban Harbour to estuarine fishes in the region, and yielding data on species that otherwise would not have been documented.
Many of the eggs encountered, could be identified from the literature, particularly when coupled with the clues contributed by the newly hatched larvae. A small, scattered, local literature, dating back to the “father” of marine biology in South Africa, JDF Gilchrist, was conveniently summarised in the pioneering research, in the regional context, of Brownell (1979). Other useful works included Delsman (1921-1938), Uchida et al (1958), a series of papers by Mito (1961-1963), Fahay (1983), Leis & Rennis (1983), Moser et al (1984), Okiyama (1988), Matarese et al (1989), Leis & Trnski (1989), Shao et al (2001), and most recently the huge and impressive work on Atlantic fishes, edited by Richards (2006). But there were many eggs which could not be identified with any certainty. In the early stages of the study, hardy Pleuronectiformes, which could, literally, be reared in a glass bowl, gave incentive to attempt rearing of the unknown eggs, in the hopes of rearing at least one specimen to the point where fin counts and juvenile features aided in identification. Towards this end, a simple foodchain was set up, involving the green alga Chlorella, the rotifer Brachionus plicatilis and the brine shrimp Artemia.
In the latter stages of the project, it was becoming increasingly apparent that many important species were not amenable to being reared in the available facilities, with the available foodchain. While recognising that the answer probably involved DNA, most of the methods being used were not sufficiently rapid and economical for the purposes of this project. In April 2003, an article appeared in New Scientist, (Hecht, 2003) outlining a technique, using a 650 base pair section of the Cytochrome C oxidase subunit 1, of mitochondrial DNA, to differentiate between cryptic species of butterflies and a variety of other insect species. An approach to this team of researchers, led by Prof. Paul Hebert at the University of Guelph in Canada, revealed advanced plans to launch an ambitious project to “barcode” the fishes of the world. Linking with the Barcode project served two important goals. Firstly, the fishes of South Africa would be barcoded, contributing to the world-wide effort, and secondly, this would provide the adult sequences that were essential to identify my larvae from their barcode sequences. In addition, the Barcode of Life Databank (BOLD) contains a much wider spread of adult sequences from around the world, and the collection is expanding rapidly. Further details can be accessed at http://www.barcodinglife.org.
Except for
occasional samples taken in the Durban area, and off Tongaat, the study has
been conducted almost exclusively offshore of Park Rynie, some 60km south of Durban (Figure 2). This area was chosen because it has the most extensive reef in
the Durban area, is only about 5km south of the Aliwal Shoal (an area which was
being earmarked for special conservation), and has extensive sandy areas
between the reefs that ensure a rich fish diversity. It also has a relatively
safe and convenient ski-boat launch-site, although, being a surf launch out of
a protected bay, remains strongly dependent on weather and sea conditions for
safe launching (Plate 1). 
Plate 1: The launch site at Park Rynie, on a good day.
The net was an elongate cone, about 2 metres long, constructed of 300 micron aperture, monofilament netting, tapering to a detachable codend. The mouth was semi-circular in shape, with a diameter of 50 centimetres. Due to an 8.5cm elongation on each side of the arch, the mouth area was calculated as 1360cm2. A small float was attached at each corner, and a lead weight at the middle of the arch, to ensure that the net settled in the water with the bar horizontal and at the surface. On each trip to sea the net was towed behind the boat at about 1-1.5knot for 10 minutes, at a site 4-5km offshore, where the water depth was 40-50metres. From early 1994, a second sample was collected on each outing, about 500m-800m offshore. During each trip, wind and current speed and direction were recorded. Notes were kept on conditions, if unusual, such as unseasonally cold water.
From time to time a calibrated, propeller-driven flowmeter (General Oceanic Model 2030R) was used to calculate the volume of water passing through the net.
2.2 Sampling in Durban Harbour Mouth
The net was pulled along a disused quay roughly 150 metres in length, on the north bank immediately east of the small craft harbour (Plate 2, white arrows). Due to the 3m high quay-wall, a 50m length of rope was used, and the net was hauled both ways along

Plate 2: Two white arrows show the quay where Durban Harbour mouth (DHM) samples were collected.
the quay, a distance of about 300m. On the KZN coast, the daytime spring high tide occurs at about 4pm local time, every two weeks. Studies have shown that these spring tide periods are peak spawning times for estuarine fishes (eg Garratt, 1998). But in order to combine sampling with dusk, which is around 17h30 in June (winter) and 19h00 in midsummer (late December), the guide used was to sample at dusk on the day on which the afternoon high tide coincided with sunset, using South African Navy tide- and sunset-tables. A single sample was collected every 2 weeks.
2.3 Recruitment sampling at the Lovu and Mkomazi estuaries
During 2005 the opportunity arose to sample larval fish recruitment to these two key estuaries in the area (Figure 2). This allowed comparison of recruitment patterns with spawning patterns of such species as Monodactylus falciformis, Rhabdosargus holubi and mullet(LIIB9 & LIIIB7). The estuaries were sampled on afternoon incoming spring tides, targeting the inflow period as the flow became sufficient to support a net anchored in the shallow mouth. The net had a rectangular mouth of 0.8 x 1.2 metres, length of about 2 metres and mesh aperture of 500 microns. Samples were removed from the cod-end every 10 minutes. Three 10 minute samples were collected on each visit to each estuary. Due to the constraints of needing to sample the early tidal flow, the two estuaries were sampled on consecutive days. When the mouth of an estuary closed, the net was manually manoeuvred in the surf for 10 minute intervals (sea conditions permitting). Though not directly comparable, these surf samples served to show which species were present, and their relative numbers. Samples were preserved in formalin, for later counting and measurement.
2.4 Live egg sample handling in the laboratory
At sea, each sample was washed from the codend of the net into a 25 litre bucket, containing about 15 litres of seawater, and then sealed with a tight-fitting lid, for transport to the laboratory. In summer, buckets were kept cool with wet towels, to avoid accelerated egg development during transport. In the laboratory, the inshore sample was aerated, while the offshore sample was concentrated into a 500ml Pyrex bowl (12cm diameter and 6cm wall), and examined for eggs, with a dissecting microscope set on 6x magnification, and using reflected light. All eggs were removed to small bowls of clean seawater, using a range of small-bore pipettes. After searching the surface of the bowl, and especially its meniscus, the bottom of the bowl was also searched, as some eggs become less buoyant, and even sink, as they develop. All the eggs were then separated into “species” by external characteristics, and where uncertainty existed, or to check for correctness, specimens were placed in Pyrex bowls of clean seawater for hatching. It was usually possible to place at least three “species” in one bowl, by selecting obvious size or feature differences that ensured they could be recognised after hatching. The process was then repeated with the inshore sample. All this had to be completed on the evening of the day the eggs were collected, as most eggs hatched overnight. In cases where unusually high numbers of eggs were collected, the dominant species were ignored during separation. After separation of the less common eggs, the sample was fixed in formalin and the more common species counted by sub-sampling, at leisure, the following day.
A simple “key” based on the physical features of pelagic fish eggs, was used to separate eggs into basic groups. Unique alpha-numeric numbers were then assigned to each “species”, for cataloguing purposes. An extensive photo-album of eggs and early larvae was built up for each “species”, as larvae developed to the full extent allowed by the yolk and oil globule in the egg.
Due to the time constraints of the initial egg sorting process, and since most sampling was done on weekends, a small laboratory was arranged at home, and the garden shed became a simple rearing facility. Thus the project became manageable, combining egg sampling with a personal passion for fishing and SCUBA diving.
A basic foodchain was developed, comprising a single celled green alga, Chlorella, cultured by the simple expedience of keeping a few tilapia, Oreochromis mossambicus, in 5000 litre plastic ponds of seawater. These were fed chicken pellets, and the water rapidly turned green. In separate smaller tanks of about 500 litres, the rotifer Brachionus plicatilis was maintained on a mixture of yeast cells, codliver oil, highly unsaturated fatty acids and Chlorella. When a suitable batch of eggs of an unknown species was encountered in a sample, a 50 litre rectangular glass tank was prepared by being half-filled with seawater, made green by the addition of Chlorella, and seeded with a screened and washed concentrate of Brachionus. The eggs or hatched larvae, were then added. Gentle aeration was supplied from a single stone at one end of the tank. A single fluorescent light, the same length as the tank, was housed in the lid, and provided 24-hour lighting. In summer these lids were left down as a temperature of about 25°C was needed, while in winter they were raised to allow cooling to 21°C. Using this method, by the time the larvae were ready to feed, usually about 5-7 days post-hatch, the rotifer population had enjoyed rapid growth and contained a good proportion of juveniles for the fish larvae to feed on. As the rotifers multiplied in the tank, they were thinned by in situ pouring of tank-water, dipped from within a 300µm mesh net, into a 50µm mesh net. Partial water replacement, and addition of Chlorella, both as food for the rotifer and for its antibiotic properties, was also done as necessary. Tanks were skimmed, at least daily, with a sheet of paper-towel, half laid on the water and drawn across the length of the tank, to remove oils and surface scum from the tank. Every attempt was made to collect growth series from successful rearings, as voucher specimens, for research purposes, to be accessioned into the SAIAB fishes collection at Rhodes University in Grahamstown.
2.6 Photography of eggs and larvae
All photography was done through a Zeiss binocular dissecting microscope, mostly at the maximum magnification of 40x, using 10x eyepieces. A single lens reflex camera, with the lens removed, and attached close up to the microscope eyepiece with a piece of plastic tubing and lens adaptor bayonet fitting, was light, quick and simple to use. Focussing was through the camera eyepiece and mirror. Three flashes were used, to avoid shake, one set off by the button on the housing, the other two by slave, while the camera was briefly opened on B setting. All pigment patterns were found to be best observed using reflected light, with the eggs in small watch glasses. Both black and white backgrounds were useful, though black was preferred unless black pigment was being highlighted. Larvae were transferred to eyeglasses, using a wide-bore pipette, and then anaesthetised with a tiny crystal of MS222, before drawing the water level down, with a fine pipette, to induce the larva to lie on its side. For myomere highlighting in larvae, aligning the larva along a white and black edge (white sheet of paper under the bowl, on a black background) was helpful. This also worked well when looking for segmentation in the yolk, tinting in the oil globule and black pigments in the egg. Recently the system was converted to taking direct digital pictures, with the camera on Macro setting, and using a ring-light for all-round illumination. Simple plastic tubing attachments allowed photos to be taken as quickly as with the previous setup, an essential feature when working with anaesthetised specimens. Rapid irrigation with clean seawater revived all but the most sensitive specimens if the anaesthetic dose was not too strong.
2.7 DNA collection and analysis
For DNA sequencing, eggs were hatched as usual, and once larvae had fully pigmented eyes, they were anaesthetised with MS222, then photographed, prior to fixing in 98% alcohol. Individual larvae were placed in Matrix box vials, in 98% alcohol, in preparation for barcoding. Tissue samples from locally collected adult fish, were also sent for barcoding, to provide sequences that could be compared with hatched larvae sequences, thereby identifying eggs. Within BOLD, sequences of marine fishes from around the world provide further opportunities to find a sequence match, for species that have not yet been sequenced from local waters. The CO1 process is described in Hebert et. al. (2003). For more information on the barcoding initiative, visit http://www.barcodinglife.org.
3. The Physical and Oceanographic Conditions in the study area
An excellent review of the climate and weather patterns of the area can be found in Hunter (1988). What follows is a brief summary of those aspects considered relevant to the spawning study.
The climate of the KwaZulu-Natal coastal belt is generally described as humid subtropical with a warm summer. The coastline is fairly straight (Figures 1 and 2), with a slight slope, when viewed from north to south, of about 30° to the west.
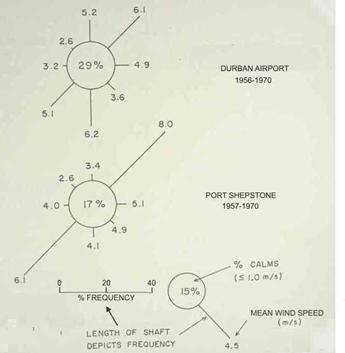
The two dominant winds are from the northeast and southwest, and they are both essentially parallel to the coast. In the Durban to Park Rynie area, they occur about equally often in the summer (Figure 3). During winter, the south and southwest winds tend to be a little more frequent, and stronger (Figure 4). These southwest winds are associated with coastal lows (known locally as westerly busters), usually preceding a cold front, and the cold front itself. The generation of these coastal lows is well described by Hunter (1988).
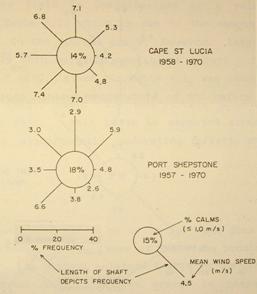
The presence of the warm Agulhas Current offshore, and the rapid cooling of the hinterland at sunset, results in the land breeze becoming a nightly affair on quiet nights, especially during the winter months (Figure 4), when it is even strong enough to occlude the northeasterly during the night. Offshore this is also manifested as a dense bank of cloud over the Agulhas Current in winter. Hunter (1988) reported that the land breeze usually dissipates by 08h00, but in
Figure 3: Average wind conditions in December (from Hunter, 1988).
mid-winter, it often blows until 11h00 (A Connell, personal observation). The incidence of summer sea breezes, on the other hand, is reduced by the warm offshore current on the KZN coast (Preston-White 1969).
The annual rainfall along the coastal belt is between 1000 and 1100mm. It is a summer rainfall area, with above 70% falling in the summer months of November to March. Due to the high escarpment adjacent to the coast, most rivers have deeply cut courses, and the Mkomazi river, just north of the
Figure 4: Average wind conditions in June (from Hunter, 1988). But note that the wind rose for Durban has been replaced by one for Cape St Lucia (see Figure 2) because Durban Airport is acknowledged as poorly placed to adequately measure the incidence of offshore winds (Lundie 1979).
study area, extends to the mountainous escarpment some 150km from the coast. Although not a massive river, it has a MAR (mean annual runoff) of 1036x106 cu.m. High erosion rates in the hinterland cause frequent discolouration of coastal waters in the summer. Most of the shorter rivers have small coastal lagoons, confined between short, sand filled coastal floodplains and a sandbar across the mouth. They often only breach once or twice in the summer, or not at all during years of poor rains (Begg, 1978). During wet summers, tongues of silt-laden river water are a common feature, penetrating a kilometre or more into the sea adjacent to the mouth of the bigger rivers such as the Lovu (MAR 112x106cu.m.) and Mkomazi.
3.4 Coastal Oceanography
The KZN coast has a relatively narrow shelf (Figure 2), the 200m depth contour being within 20km of the coast for most of its length, and this is true of the Park Rynie area. Tide range is at most about 2m at peak spring tide, down to about 0.5m at neap tides.
The dominant oceanographic feature along the entire KZN coastline, is the Agulhas current, one of the major western boundary currents of the world. A review of the coastal oceanography of the area can be found in Schumann (1988). Water temperature in the current core, rises to about 28°C in summer, and drops to about 23°C in winter.
On average the Agulhas current is 40-60km offshore in the vicinity of Durban (Schumann 1988), but the inner edge can change its position by 30km and more in a day. Such meanders can be seen in Plates 4-8. Off Port Edward, Schumann (1988) reported that the Agulhas current was usually so close inshore that the inside edge, indicated by a sudden temperature rise of about 2°C, was seldom discernable,
3.4.2 Inshore waters and currents
Being a western boundary current, travelling south in the southern hemisphere, the Coreolis effect, also termed Ekman sheer, causes Agulhas current midwater to slide onto the shelf all year round (Pearce, 1978; Schumann 1988). This water is reported by Pearce (1978), to be from 40-60m depth in the Agulhas current, and is on average about 1.4°C cooler than Agulhas current surface water. In summer the surface of this cooler inshore water is rapidly warmed by the sun, resulting in a marked difference (about 4°C, according to Pearce, 1977), between surface and bottom temperature in shelf water 30-50m deep. Recent data from a thermister string deployed in 31m off Amanzimtoti demonstrates this (Figure 5). In winter, the weaker sun, along with the nightly cooling effect of the winter landbreeze, prevents this temperature difference from developing. Thus, in winter, inshore shelf water temperatures are generally within about 1°C from surface to bottom, at around 21°C (Figure 5). Countless satellite images
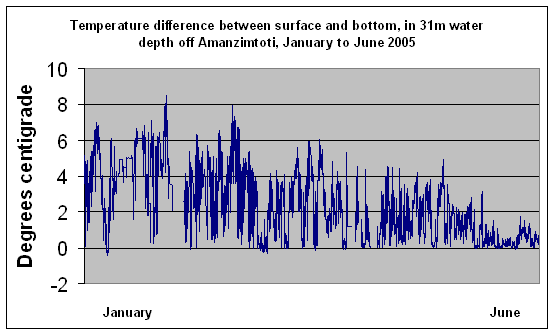 |
Figure 5.(Data courtesy J. Howlett, Huntsman Tioxide) |
confirm the presence of cooler water inshore of the Agulhas current, as shown in the two examples in Plate 3 below.
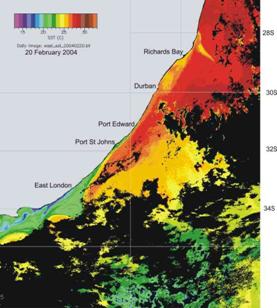
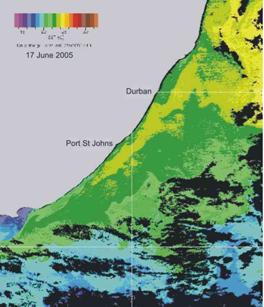
Plate 3. Sea surface temperature images, summer (left), and winter (right), illustrating the presence of a band of cooler inshore water, relative to the Agulhas current, throughout the year along the KZN coast.
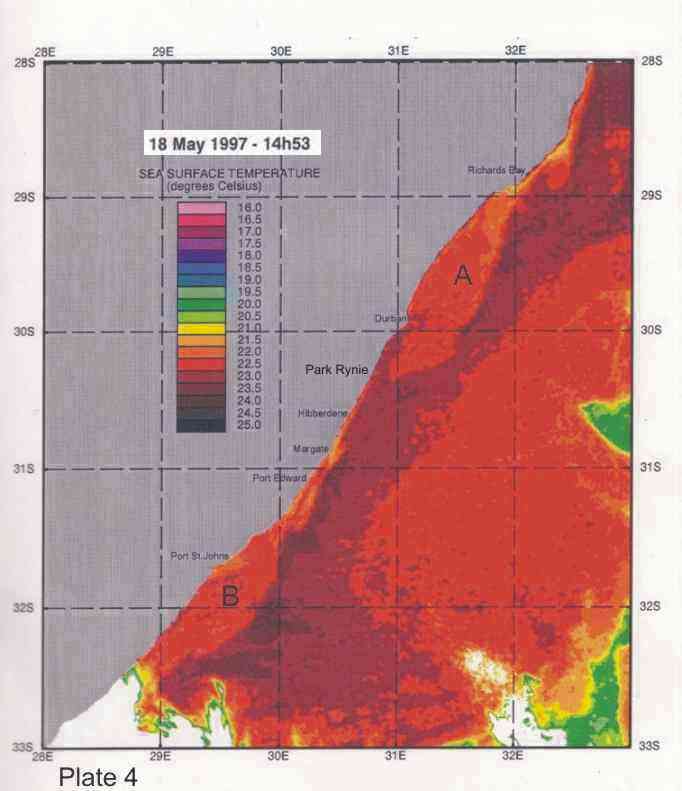
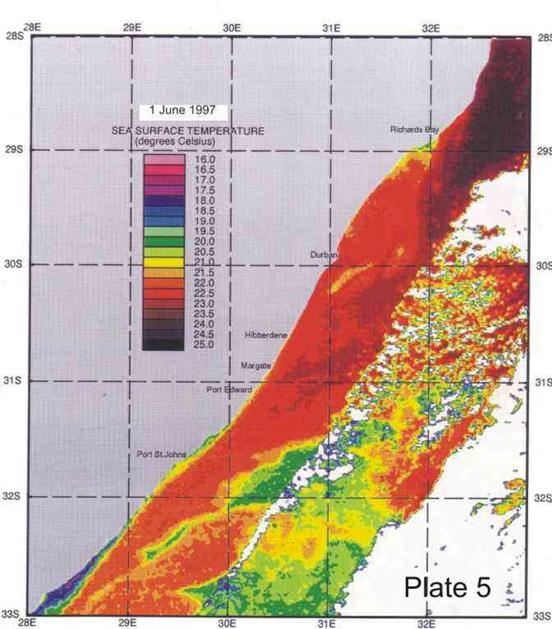
Inshore currents are predominantly wind-driven, outside of the influence of topographically driven features such as the Durban-Richards Bay (A in Plate 4) and Port St Johns Bights (B in Plate 4). Although caused by relatively small kinks in the coastline, these are the sites of semi-permanent eddies of expanded cooler inshore water, with predominantly north-going currents on their inshore edges. Inshore currents in the Park Rynie area are largely wind driven, but complicated by southern extensions of the Durban Bight eddy, as is clearly demonstrated in Plate 4. This is confirmed by the unusual incidence of inshore currents off Park Rynie, reported by Schumann (1988). The Durban Shelf area is described by Schumann, (1988) as “a transition region between the wind-dominated shelf to the north, and the Agulhas Current dominated shelf to the south”, with evidence
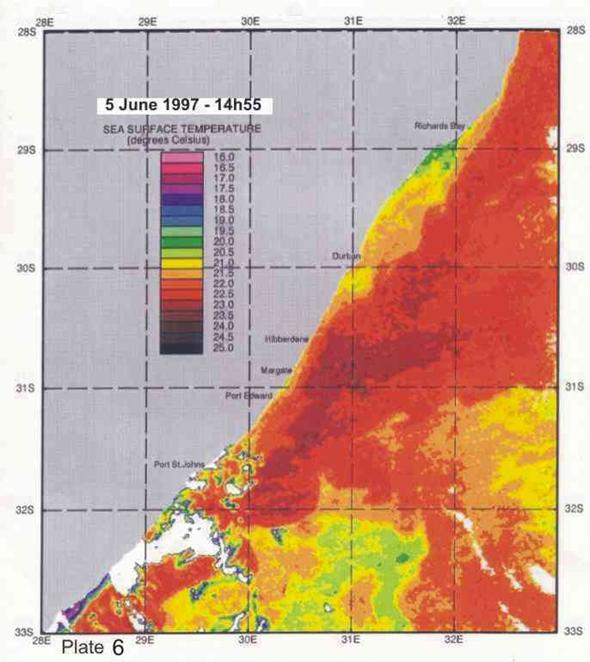
that the area between Durban and Park Rynie (his Mzinto) often shares the same water in a recirculating eddy.
A further disrupting feature to inshore currents, is
the passage of a “Natal pulse”, essentially a moving eddy of water trapped
inside the Agulhas Current. A “pulse” appears to be generated when a
topographically generated eddy becomes unstable and breaks free to move down
the coast (or a large inshore deflection of the Agulhas current becoming
isolated), with a rapid clockwise rotation (when viewed from above), generating
unusually powerful north-going currents on its inshore edge (Lutjeharms and Connell 1989). The
big eddy off Hibberdene in Plate 7 is probably an example of this feature. A
“pulse” moves down the coast at a speed of about 22km per day (Lutjeharms and Roberts 1988),
and, due to their size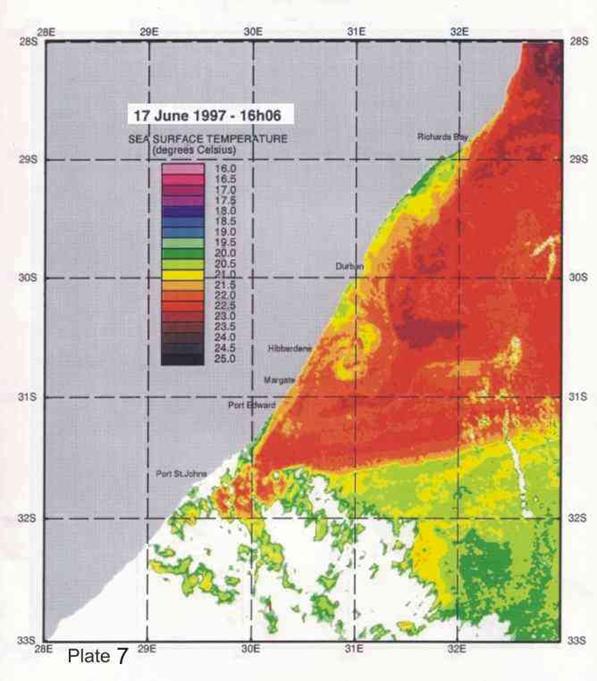
and depth, can be associated with cold water, and unusual, deepwater fauna being pulled up onto the shelf (eg squid egg balloons, and deepwater copepods). Most studies seem to suggest that these “pulses” are relatively infrequent, with only 3-4 passing through each year. The coldest water we ever encountered during the 25 years of diving related to this research, was 14.5°C, on 5 February 2006. That this was deep offshore water being pulled onto the shelf by a "Natal pulse", is supported by our collecting the first record of squid egg balloons from the east coast of South Africa (Roberts, Zemlak and Connell 2011). Such water would come from >150m over the shelf edge (Schumann 1988).
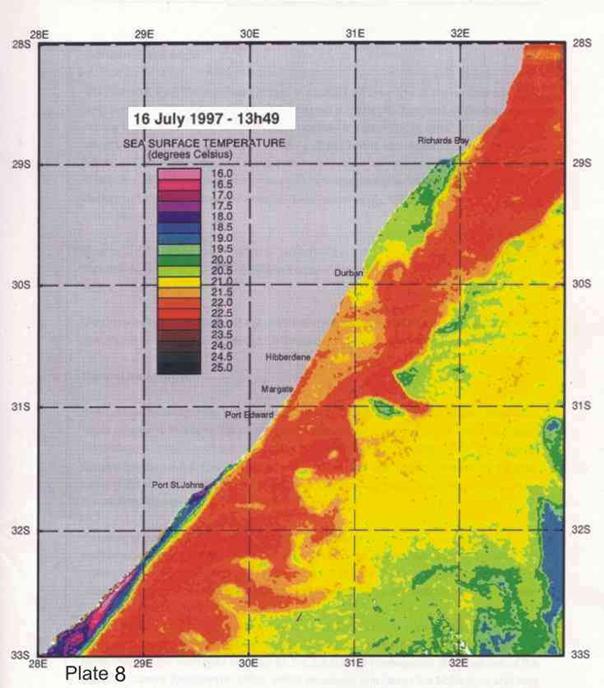
Plate 4 shows a band of cooler inshore water occupying
the entire bight from Durban to just south of Richards Bay, caused by the
Agulhas current being confined further offshore by the straight shelf break (Figure
2), with the coastline cutting away to the west, immediately south of Richards Bay. Flemming and Hay (1988) showed how bottom currents inferred from sediment
dispersal and bedform patterns revealed a complex flow (Figure 6). These
confirmed a closed eddy system off the Tugela mouth, and an accompanying
depocentre for fine 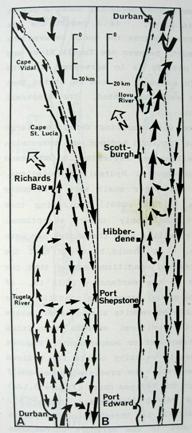 silts, as well as a semi-permanent (also cyclonic)
eddy just north of Durban. In Plate 4 a similar eddy can be seen, centred just
south of Port St Johns, extending north to about where 30°E longitude cuts the coast. For fishes moving up the
coast on annual migrations, these two semi-permanent eddys form important
“stepping stones”, since their inshore edges have predominantly north-going currents.
silts, as well as a semi-permanent (also cyclonic)
eddy just north of Durban. In Plate 4 a similar eddy can be seen, centred just
south of Port St Johns, extending north to about where 30°E longitude cuts the coast. For fishes moving up the
coast on annual migrations, these two semi-permanent eddys form important
“stepping stones”, since their inshore edges have predominantly north-going currents.
3.4.4 Coastal productivity, nutrients, zooplankton and fish
 The Agulhas Current, being the dominant
feature of the coastal oceanography of this region, also plays a major role in
the year-long nutrient loading and productivity of the region. Studies have
shown that surface water has a negative gradient in nutrient concentration with distance
offshore as far as the Agulhas Current core [Oliff (1973), and Pearce (1977a)
in Carter and D’Aubrey (1988)], as well as a positive gradient with depth in
the Agulhas Current itself. The continuous Ekman veering of Agulhas Current
midwater up onto the shelf, results in the inshore waters being cooler, and slightly
elevated in inorganic nutrients, (N, P and
Si), compared to the Agulhas Current core surface water.
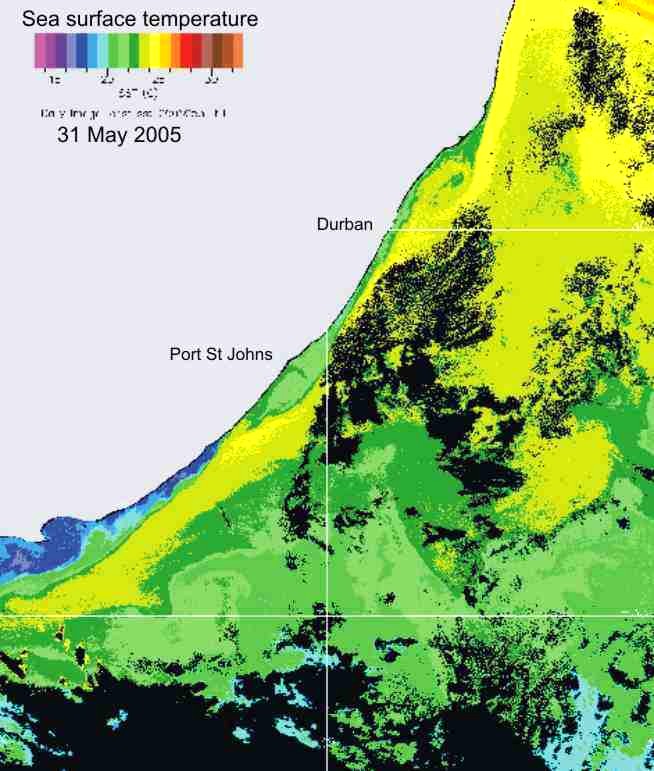
In KZN inshore waters, there is
thus a strong correlation between the cooler inshore water, and chlorophyll-a
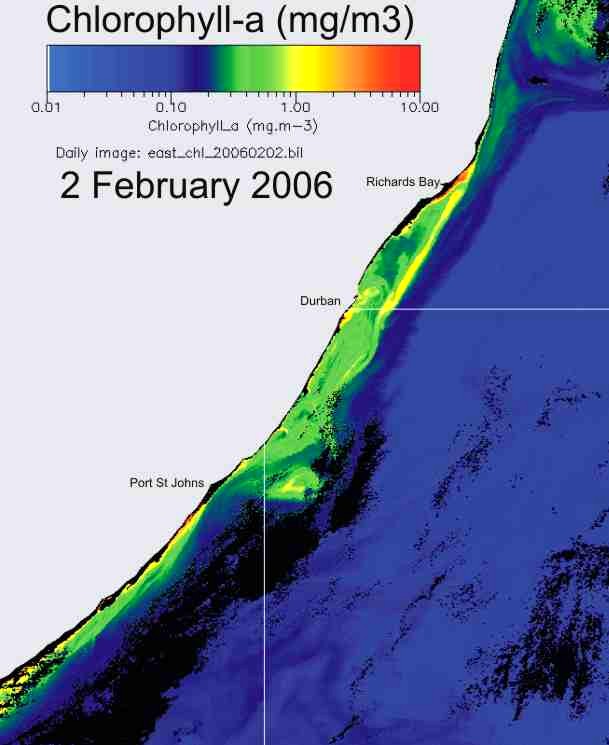
production (Plate 9), and the chlorophyll-a levels are similar in summer and
winter (Plate 10).
The Agulhas Current, being the dominant
feature of the coastal oceanography of this region, also plays a major role in
the year-long nutrient loading and productivity of the region. Studies have
shown that surface water has a negative gradient in nutrient concentration with distance
offshore as far as the Agulhas Current core [Oliff (1973), and Pearce (1977a)
in Carter and D’Aubrey (1988)], as well as a positive gradient with depth in
the Agulhas Current itself. The continuous Ekman veering of Agulhas Current
midwater up onto the shelf, results in the inshore waters being cooler, and slightly
elevated in inorganic nutrients, (N, P and
Si), compared to the Agulhas Current core surface water.
In KZN inshore waters, there is
thus a strong correlation between the cooler inshore water, and chlorophyll-a
production (Plate 9), and the chlorophyll-a levels are similar in summer and
winter (Plate 10).
 |
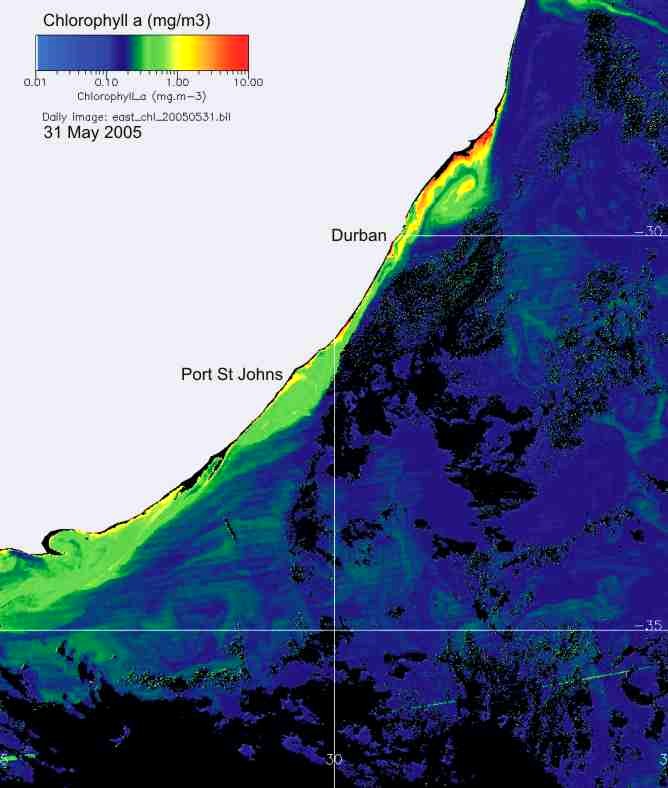 |
Plate 9. Demonstrating the strong relationship between the cool inshore water, and chlorophyll-a concentration, along the KwaZulu-Natal coast.
|
 |
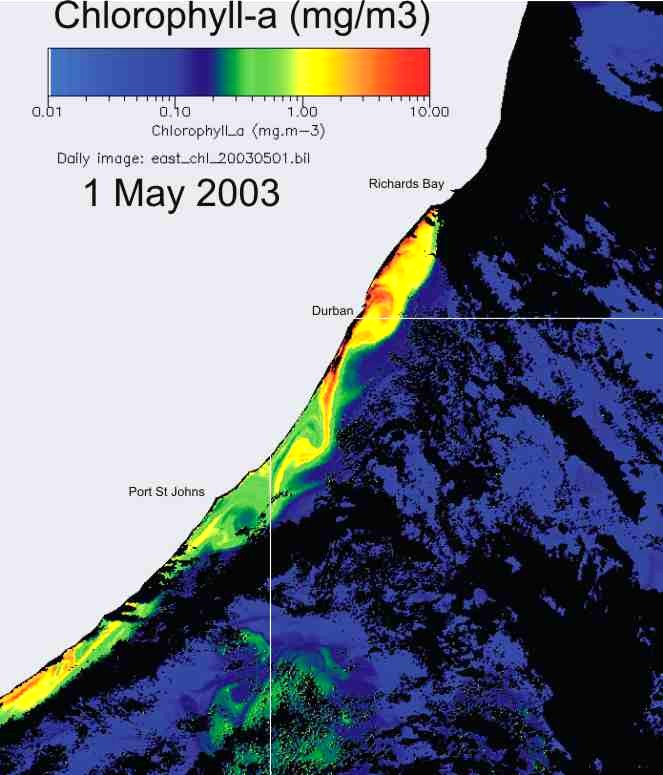 |
Plate 10. Comparison of chlorophyll-a concentration, in summer (left) and winter (right), along the KwaZulu-Natal coast. |
Zooplankton has not been extensively studied on the KZN coast, and nothing has been added since the review of Carter and Schleyer (1988), apart from papers on fish larvae, which are not relevant to the present discussion. By far the dominant animals in most sea surface zooplankton samples are the copepods, and they are also the key component in the food web from phytoplankton to fishes. Transects across the Agulhas current have shown a higher biomass inshore of the current (Carter 1977), and the bulk of the biomass (70%) in the upper 100m (Carter and Schleyer 1988). The dominant species in the core of the current was Paracalanus parvus, but on the shelf, Centropages chierchiae and Calanoides carinatus were dominant (Carter 1977). Carter (1977) recorded a shelf biomass minimum in the Durban area, in the late summer (March), and a winter biomass bloom dominated by the large calanoid, Calanoides carinatus. This is a key species in the diet of sardine; thus a winter bloom would be an important food source for this species during its annual winter migration to KZN waters. During the present study, notes were kept when big samples of large copepods were encountered in the egg samples, and by far the most common large copepod seen in winter, was Calanus agulhensis, also important in the diet of sardine.
Productive food chains, which in coastal seas lead to productive fisheries, are obviously dependent on sustained high primary production (phytoplankton), driven by nutrients, which are usually brought to the surface in cold upwelled water. This in turn supports a rich zooplankton, feeding on the phytoplankton bloom, and supporting fast-growing, pelagic, shoaling fishes such as sardines and anchovies. This is clearly not the case in KZN, due to the oligotrophic nature of the shelf waters, despite the Eckman-veer driven upwelling, and small areas of increased local upwelling such as the semi-permanent eddy located east of Durban, which can cause significant upwelling of nutrient richer, cold water at its centre (Pearce 1979), and the inshore waters south-west of Durnford Point (Lutjeharms et al 2000). The point is best illustrated by comparing the coastal fisheries of the South African coast. The KZN shelf does not support any year-round pelagic fishery, and only yields between 1500 and 2000 tonnes of linefish and about 500 tonnes of shoaling sardine-like pelagics, these latter associated with the annual winter “sardine-run” up the east coast from Cape waters (van der Elst 1988). By contrast, the southern Cape coast fishery yields in the region of 100 000 tonnes annually, and the western Cape fishery in the region of 600 000 tonnes annually. Without the zero’s the ratio is 2:100:600. Clearly the east coast is not a significant fishery. The real value of the KZN fishery, in the local context, is the quality of the fish harvested (van der Elst, 1988), which finds its way into local markets and onto local tables, via a small but active commercial fishery and an ever growing recreational fishery. Many of the fish species are of tropical origin; the warm, powerful Agulhas Current allows tropical species to extend their range further south than would otherwise be the case. But the area also exhibits a high degree of endemism, due again in no small measure to the powerful Agulhas Current which, along with the vast and open southern Indian Ocean, forms an effective barrier to coastal species migration, away from the southern tip of Africa.
4. Coastal oceanography in relation to sardine movement into KZN waters in winter
The Agulhas Current plays a major role in shaping the so-called Natal sardine run each year. There is no evidence of a winter counter-current up the east coast, as has often been suggested in popular articles about the “Natal sardine run”. At all times of the year, shelf waters are a couple of degrees cooler than Agulhas current water. As winter approaches, inshore waters, continuously fed by Ekman veering of Agulhas current midwater (Section 3.4.2), gradually cool. As the sardine shoals move up the coast, in this cooler inshore water, they encounter, in the vicinity of Port St Johns, a narrowing of the shelf towards Port Edward (Plate 4), and consequently a narrowing of the band of cooler water between the Agulhas current and the coast. In this area there is evidence of an eddy formed in a similar way to the cooler water between Richards Bay and Durban (see Section 3.4.3), which ends in a bottleneck off Port Edward (Plates 4 - 8). While not consistently as wide as the Durban to Richards Bay complex of eddies, the slight westward slope of the coast south of Port Edward appears to contribute to this feature (Plates 3-10). Within this zone, the clockwise rotation of an eddy creates north-going inshore currents, which would assist the northward movement of the shoals. The Port Edward bottleneck, created by the very narrow shelf in that area (Schumann 1988) ensures that the shoals gather in the northern extremity of the eddy, ever closer to the coast. In some years the shoals get pushed through the bottleneck, by a severe cold front from the south-west, with strong southwesterly winds, spoiling the spectacle of the sardine shoals on the lower south coast of KZN. But in calmer years, the shoals will amass until the migratory imperative forces them to gather into a larger shoal which proceeds up the coast, hugging the coast to avoid the Agulhas Current offshore, in a long thin shoal that is often only about 50m wide, but can stretch 15-20km or more along the coast. If strong southerly currents are encountered just offshore, the “main” shoal can move in this fashion as far as Hibberdene or even Park Rynie, and has, occasionally, as on 30 June 2005 (Plate 11), even reached Durban in this mode.
 |
Plate 11. Taken at Brighton Beach, Durban, on 30 June 2005, this image shows a thin continuous band of sardines (light purple in a blue sea), just behind the breaking wave. The shoal stretched for about 15km along the coast, at the time this picture was taken. The white dots and rafts in the background are seabirds (Cape Gannet), resting on the water after feeding on the sardines earlier in the morning. |
Generally, however, they will break up into smaller shoals further down the coast, and continue moving north. Once under the influence of the predominantly north-going currents north of Durban, the sardines tend to disperse further offshore, where they feed and spawn in the cooler coastal waters inside the Agulhas Current, over the next few months (Connell 2001). As can be seen in Plate 4, another significant “bottleneck” is found north of Richards Bay, and although sardines have been found spawning off Richards Bay in October (Connell 1997a, 1998, 2003), there are no confirmed reports of shoals moving through the Cape St Lucia area. Connell (1997) showed, from evidence of egg collections off Park Rynie, that sardines move back south, through the Park Rynie area, from October to December each year, as KZN coastal waters become too warm. But due to the warmer surface waters inshore, they are not seen on the surface, and only the eggs indicate their presence as they move through the area (see notes, Sardinops sagax).
5. Larval fish movement in relation to the Agulhas Current.
A major feature of spawning patterns of fishes on the shelf of KZN, is that some of the key spawning species move into these waters, from the south, in winter, on spawning migrations (van der Elst 1988, Hutchings et al 2003). The most spectacular of these are the sardines, Sardinops sagax, the sciaenids Atractoscion aequidens (geelbek) and Argyrosomus japonicus (kob); the sparids Polysteganus undulosus (seventyfour), Petrus rupestrus (steenbras), Cymatoceps nasutus (poenskop), Sparodon durbanensis (brusher), Rhabdosargus holubi (silverbream) and Sarpa salpa (karanteen), the shad Pomatomus saltatrix and the carangid Seriola lalandi (yellowtail) (van der Elst 1988, Hutchings et al 2003). Although early papers on the subject assumed that most of these species spawned out in the Agulhas Current, as a means of moving their larvae south (Heydorn et al 1976, van der Elst 1988), more detailed studies have shown this not to be the case. For example Beckley and Connell (1998), found that the elf spawned on the shelf, in 30-50m water depth, about 5km offshore at Park Rynie (some 25-30km inside the inner edge of the Agulhas Current). See the Pomatomus sheet for more information on shad egg distribution across the shelf from the present study.
Each species has a spawning strategy, together with early juvenile behaviour which, in combination, affords the juveniles the opportunity to recruit to their preferred nursery areas. The ability of very small juveniles of marine fishes, to move deliberately towards preferred habitat, is well established (Leis 2002). Regarding local species, nursery grounds are known to be in Cape littoral waters, for seventyfour (Ahrens, 1964), geelbek (Griffiths and Hecht 1995), karanteen (Joubert, 1981) and shad (van der Elst, 1981). A comparison of strategies in three local species, blacktail (LIIIC1), karanteen (LIIIB9) and silverbream (LIIID6), serve to illustrate the point. The present study has shown that karanteen spawn close inshore, predominantly within 500m of the shore (species sheet LIIIB9). Blacktail spawn both close inshore and on the further offshore reefs, including Aliwal Shoal (Figure 12). The spawning locality of silverbream is uncertain, since only 5 specimens were reared during this study, each as a single fish which emerged from batches of juveniles reared from eggs of blacktail and sand soldier (LIIIC5). See sheet LIIID6 for details.
Despite spawning close inshore, karanteen juveniles of 15-25mm SL have never been found in the rockpools, protected bays or estuaries of KZN. They are commonly found in bays of the eastern Cape (Joubert, 1981; Beckley, 1986). In the latter study, Beckley collected only 7 specimens, measuring 15.8-36.5mm SL. While the upper size limit might be the avoidance threshold of the net used, the lower limit is a fish in excess of 30days old (see data sheet LIIIB9). This allows that Beckley's specimens could have come from KZN spawning (note however that Strydom and d'Hotman (2005) collected juveniles as small as 8.9mm SL in the surf at Cape Padrone, at the eastern corner of Algoa Bay, indicating that spawning also occurs in eastern Cape waters). In the present study, juvenile karanteen from offshore plankton and handnet catches, have only been recognised on three occasions, twice being small specimens from the plankton net, while towing for eggs. But on one occasion, when quiet calm conditions prevailed, a loose shoal of small fish using the skiboat hull as shelter, proved to be karanteen juveniles. Details are shown in the following table:
|
Date |
n* |
Size range (SL) |
Distance Offshore |
Water depth (m) |
|
9/11/2003 5/8/2004 8/8/2004 |
1 9 (40) 1 |
10.5mm 27.1-29.0mm 13.6mm |
800m 4km 800m |
15m 38m 15m |
* Number caught. In brackets is the estimated number in the shoal.
The shoaling behaviour seen on 5 August 2004 might suggest that congregating at the surface is a successful strategy for juveniles to move south into Eastern Cape waters. Given that inshore currents are wind dominated, and strongest at the surface, the high incidence of northeasterly winds during August to October along the KZN and Eastern Cape coasts, would contribute to south-westward transport of surface shoaling juveniles.
Adult blacktail tend to spawn a little further offshore, but inside the 30m depth contour ( LIIIC1), and their eggs were also common in the Durban Harbour mouth samples. Small juvenile blacktail abound in rocky embayments, protected reefs in the surf, and intertidal rock pools, all along the southern KZN coast in early summer (Joubert 1981). Thus, early juvenile behaviour is to make for these areas, which act as nurseries for this species.
The deliberate movement of juvenile silverbream is perhaps the most remarkable of the three. Adults are caught on deeper offshore reefs in KZN (Smith & Smith 1986), suggesting this is where they spawn, supported by five reared specimens from this study, mentioned above. But their early juveniles enter estuaries as their preferred nursery area (Wallace & van der Elst 1975). In the present study the larvae have been found to recruit to the Lovu and Mkomazi estuaries virtually all year round (Figure 7), at a constant 9.5-11mm SL, which, judging from
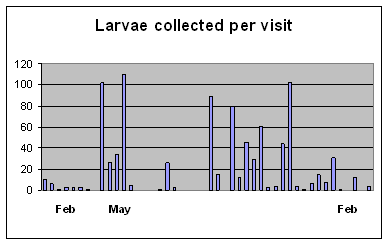 |
| Figure 7. Recruitment pattern of Rhabdosargus holubi larvae to the Mkomazi and Lovu estuaries, from February 2005 to February 2006, sampled two-weekly on incoming spring tides. |
rearing of related species such as Diplodus capensis, Rhabdosargus sarba, Sarpa salpa and Crenidens crenidens, would represent a fish of 30-35 days old. While attempting to recruit to the closed Lovu estuary, these tiny juveniles were found in the turbulent surf opposite the estuary, and even in waves running back down the sandbar’s seaward slope after failing to overtop into the estuary (Plate12). During a genuine overtopping
Plate 12; Netting for fish larvae in receding waves, adjacent to the closed mouth of the Lovu estuary. |
event on 11 September 2002, the same net was used, and collected 5 tiny silverbream along with 8 juvenile mugilids (11-12mm SL) by netting on the estuary side of the berm, in intermittently overtopping, shallow waves (Plate 13). The deliberate presence of these estuary-recruiting species in the surf
 |
Plate 13. High spring tide overtopping of waves across the berm into the Lovu estuary during a period of mouth closure. |
adjacent to the estuary is demonstrated by their dominance in catches in the surf at the Lovu estuary during 2005, while the mouth was closed. About 90% of the fish larvae collected, were species that actively recruit to estuaries, and 80% were silverbream, Monodactylus sp. and mugilid juveniles combined (A Connell, unpublished). Cowley et. al. (2001) have reported similar deliberate recruitment by overtopping into closed Eastern Cape estuaries.
6. Rainfall and spawning intensity
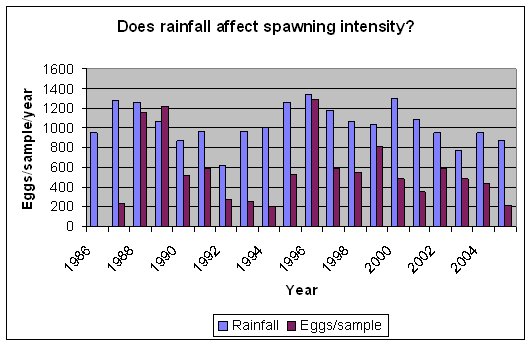
During September 1987, a flood event of extreme proportions occurred, during which up to 800mm of rain fell in a period of 5 days (Badenhorst et. al. 1989). The bigger rivers had flood duration periods of up to 24 hours, and divers working on an offshore pipeline, 3km off the mouth of the Mkomazi river, reported the seabed rising over 1m at the diffuser. In dives off Park Rynie, we also recorded mud lying 15cm deep in gutters in the reef at 50m depth, about 5km offshore. The nutrient load from such a deluge has never been calculated, but a striking feature of the following few years of this study was that they yielded unusually high numbers of eggs, particularly from the three most prolific pelagic egg spawners, namely the sardine, Sardinops sagax, the East coast roundherring, Etrumeus teres and the mackerel, Scomber japonicus (Connell 2001). Due to the huge variability in eggs-per-sample from this study (0 – 46235), and the enormous difference to an annual mean that a single large sample can make, the statistics are questionable. Nevertheless, a comparison of rainfall and spawning intensity, over the period of the study, does show a similar pattern (Figure 8), with an expected lag of a year, given the summer rainfall pattern and the winter/spring spawning maximum (Figure 9). Isotope studies on eggs and zooplankton are currently underway, in an effort to confirm the link.
 |
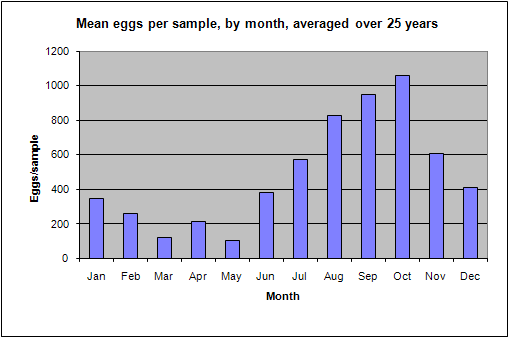 |
| Figure 8: Rainfall in the Mkomazi catchment, versus mean annual egg per sample | Figure 9: Mean monthly eggs per sample, averaged over 25 years. |
7. Results
7.1 Dive log current data for the study area
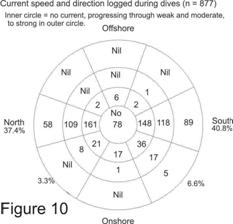 Of 877 dives on which current speed and
direction were logged, the number of days and percentage of 8 segments of the
compass rose are shown in Figure 10. (Note that for simplicity this chart had
been drawn as though the coast was aligned with True North, whereas it is in
fact about 30°E of TN. Thus those currents running in the segment denoted as
“north” are actually running 30°NNE, approximately parallel to the
coastline, and the “south” segment is running 210°SSW). Note also
that, for obvious reasons, the data is biased towards days when weather
conditions favoured safe launching and diving.
Of 877 dives on which current speed and
direction were logged, the number of days and percentage of 8 segments of the
compass rose are shown in Figure 10. (Note that for simplicity this chart had
been drawn as though the coast was aligned with True North, whereas it is in
fact about 30°E of TN. Thus those currents running in the segment denoted as
“north” are actually running 30°NNE, approximately parallel to the
coastline, and the “south” segment is running 210°SSW). Note also
that, for obvious reasons, the data is biased towards days when weather
conditions favoured safe launching and diving.
7.2 Net volume and eggs per sample
From time to time, a propeller driven counter was used to calibrate the volume of water passing through the net. The results are summarised in the following table.
|
Number of records |
Range (m3/sample) |
Mean (m3/sample) |
Standard Deviation |
|
110 |
22.8 – 99.2 |
59.1 |
17.6 |
The high variability is due both to clogging of the net, variable sea conditions, and possibly, among the low values, some unseen interference with the propeller (i.e. it under-counted) during sampling.
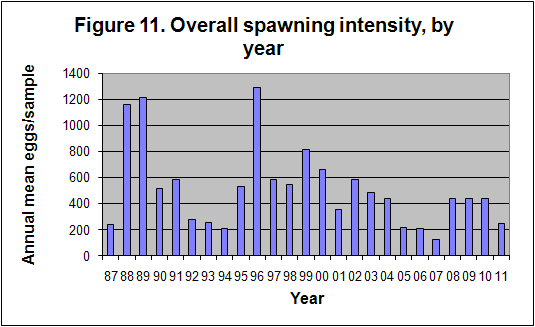 The mean number of eggs per sample has, as
mentioned in Section 6, shown a trend which appears to follow rainfall, but has
essentially hinged on the successful movement into the area, of actively
spawning shoals of fishes which include the sardine, east coast round herring,
mackerel, the maasbanker Trachurus trachurus, a variety of Decapterus
species (scads), including D. macrosoma and D. russelli, and an
anchovy (BDIIIA1). The overall trend is shown in Figure 11, while the
seasonal trend in spawning is shown in Figure 9 above. The months of August to
October are the peak spawning months, when the three most abundant spawners, as
well as most of the sparids, particularly the sandsoldier Pagellus
natalensis, are spawning (see individual species sheets).
The mean number of eggs per sample has, as
mentioned in Section 6, shown a trend which appears to follow rainfall, but has
essentially hinged on the successful movement into the area, of actively
spawning shoals of fishes which include the sardine, east coast round herring,
mackerel, the maasbanker Trachurus trachurus, a variety of Decapterus
species (scads), including D. macrosoma and D. russelli, and an
anchovy (BDIIIA1). The overall trend is shown in Figure 11, while the
seasonal trend in spawning is shown in Figure 9 above. The months of August to
October are the peak spawning months, when the three most abundant spawners, as
well as most of the sparids, particularly the sandsoldier Pagellus
natalensis, are spawning (see individual species sheets).
7.3 Offshore/Inshore sample analysis
As noted in the Section 2.1 of Methods, from early
1994 a second, inshore sample was collected on each launch. Comparison of the
individual species distribution of eggs in the offshore and inshore samples (i.e. collected on the same day, and referred to as linked samples), gave an indication of which species spawned closer inshore (500m
offshore compared with 4-5km offshore). These data are presented in a small
data box on individual species sheets, with the number of samples containing at
least one egg of the species, and the total number of eggs. The data from the
two sample sets is not directly comparable, due to a significantly different
mean number of eggs per sample, which for the offshore set, is 584, and inshore,
380 (n = 544). Reasons for this would include the obvious lack of an inshore source
for the inshore sample, and higher diversity and biomass on offshore reefs.
When considering individual species, and where they spawn, comparison of
percentage occurrence in the two, linked sample sets provides interesting data (Table
1). Two indicator species were used to gain a relative idea of where each
species spawns. The first is the kob Argyrosomus japonicus, which forms 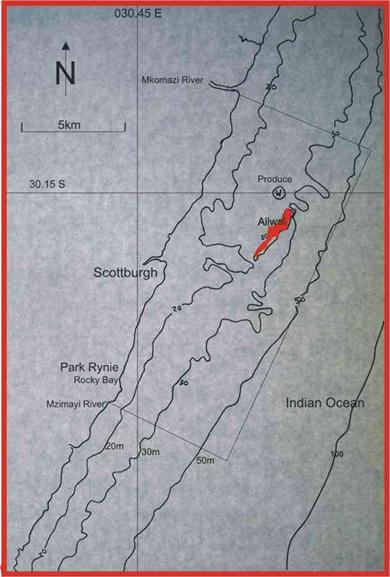 spawning aggregations on
wrecks in the area, including the Produce in 30m water depth, less than 1km NW
of the Aliwal Shoal (Figure 12), and the Griqualand, in 50m water depth about
15km south of Durban (Figure 2). The other is the geelbek Atractoscion
aequidens, which forms large spawning aggregations on reefs off Park Rynie.
While local commercial fishermen report that they used to be caught on reefs
at 30m, they currently are mostly caught at 45-60m water depth, slightly
further offshore than kob. Table 1 lists the data for all the linked samples,
from the species with the highest inshore percentage at the top. Top of the list is Monodactylus falciformis,
which spawns in close proximity to the surfzone, and can often be
spawning aggregations on
wrecks in the area, including the Produce in 30m water depth, less than 1km NW
of the Aliwal Shoal (Figure 12), and the Griqualand, in 50m water depth about
15km south of Durban (Figure 2). The other is the geelbek Atractoscion
aequidens, which forms large spawning aggregations on reefs off Park Rynie.
While local commercial fishermen report that they used to be caught on reefs
at 30m, they currently are mostly caught at 45-60m water depth, slightly
further offshore than kob. Table 1 lists the data for all the linked samples,
from the species with the highest inshore percentage at the top. Top of the list is Monodactylus falciformis,
which spawns in close proximity to the surfzone, and can often be
Figure 12. Aliwal Shoal and the Produce wreck, in relation to Park Rynie and the 20m, 30m and 50m depth contours. The rectangle shows the boundaries of the Aliwal MPA. |
seen feeding in loose shoals in the surfzone. The two indicator species discussed above, are highlighted in green in Table 1. In the species pages which follow, spawning is discussed in relation to the two indicator species, and the depth contours in Figure 12.
Table 1: Percentage of eggs in samples from 5km (offshore), and 0.5km (inshore), for the Park Rynie linked samples, in rank order, excluding most species with <50 eggs (1987-2011)
| Code | Identification | number of eggs | % Offshore | % Inshore |
| FII A9 | Monodactylus falciformis | 2590 | 7 | 93 |
| MII A1 TypeA | Dagetichthys marginatus | 68 | 10 | 90 |
| LIIIB9 | Sarpa salpa | 6438 | 14 | 86 |
| FIIIA9 | Sillago chondropus | 58 | 16 | 84 |
| MIIIA4 | ?Apodocreedia vanderhorsti | 399 | 17 | 83 |
| EIIIB6 | Caranx sem | 192 | 17 | 83 |
| LII B6 | Umbrina robinsoni | 171 | 19 | 81 |
| MIIIA8 | Cynoglossus sp | 4712 | 20 | 80 |
| LIIIC1B | Pachymetopon aeneum + | 462 | 20 | 80 |
| LIIID9 | Rhabdosargus sarba | 634 | 21 | 79 |
| KIIIC3 | Thalassoma trilobatum | 327 | 21 | 79 |
| FIIIA2A | Dichistius multifasciatus | 165 | 23 | 77 |
| LIIIA6 | Platycephalus indicus | 105 | 24 | 76 |
| LII A5 | Aeoliscus punctulatus | 125 | 25 | 75 |
| LII B8 | Scorpaenidae | 49 | 27 | 73 |
| LIIA6A | Tripterodon orbis | 428 | 27 | 73 |
| LII B4 | Diplodus hottentotus | 333 | 28 | 72 |
| MIIIA5 | Pagusa nasuta (= Solea bleekeri) | 74 | 28 | 72 |
| LIIIC9 | Pseudorhombus arsius | 114 | 29 | 71 |
| LIIIA11A | Euthynnus affinis | 353 | 32 | 68 |
| LIIIA5 | Cociella heemstrai | 585 | 32 | 68 |
| LIIID1 | Lithognathus mormyrus | 1414 | 33 | 67 |
| EIIIB1 | Upeneus guttatus | 1708 | 34 | 66 |
| LIIIE1 | Parapercis robinsoni | 1549 | 36 | 64 |
| MIIIA7 | Limnichthys nitidus | 546 | 36 | 64 |
| LIIIB10 | Pachymetopon grande | 2455 | 37 | 63 |
| KIIIC1 | Pseudanthias fasciatus | 1785 | 40 | 60 |
| FIIIA5 | Chaetodon marleyi | 363 | 40 | 60 |
| EIIIB3 | Pomadasys striatum | 32374 | 40 | 60 |
| LII A2 | Pempheridae | 801 | 40 | 60 |
| EIIIB3A | Kuhlia mugil | 19 | 42 | 58 |
| KIIIA2 | Anchichoerops natalensis | 161 | 42 | 58 |
| LIIIC5 | Pagellus natalensis | 75730 | 42 | 58 |
| KIIIB8 | Labridae | 11869 | 43 | 57 |
| FIIIA7 | Sillago cf sihama | 119 | 43 | 57 |
| LIIIF7 | Unknown | 304 | 43 | 57 |
| KIIIA8 | Paracaesio xanthura | 1851 | 44 | 56 |
| MIIIA2 | Onigocia oligolepis & ?Thysanophrys celebica | 129 | 45 | 55 |
| CDIIIA1 | Draculo celetus | 236 | 46 | 54 |
| EIIIB4 | Pomadasys olivaceum | 2823 | 46 | 54 |
| MII A2 | Sarda orientalis | 659 | 47 | 53 |
| CHII A1 | Synodontidae | 5172 | 47 | 53 |
| FIA1 | Anguilliform | 79 | 48 | 52 |
| LIIIC1 | Diplodus capensis | 29041 | 48 | 52 |
| LIIIF5 | Secutor insidiator | 2565 | 49 | 51 |
| MIIIA1 | Rogadius portuguesus | 306 | 49 | 51 |
| LIIIF2 | Chaetodon spp. | 3855 | 49 | 51 |
| BFIIIA1 | Stolephorus holodon | 268 | 49 | 51 |
| G II A1 | Hilsa kelee | 52 | 50 | 50 |
| EIIIB8 | Gerres acinaces | 280 | 51 | 49 |
| LIIIG7 | Crossorhombus valderostratus | 15325 | 51 | 49 |
| KIIIB2 | Cyprinocirrhites polyactis | 377 | 51 | 49 |
| FIIIA2 | Dinoperca petersi | 269 | 54 | 46 |
| KIIIA4 | Serranus knysnaensis | 599 | 55 | 45 |
| EIIIA6 | Decapterus spp &Trachurus trachurus | 53082 | 56 | 44 |
| LIIIE7 | Acanthurus mata | 9134 | 56 | 44 |
| KIIIA6 | Brotula multibarbata | 1243 | 56 | 44 |
| CMI A2 | Zebrais regani | 75 | 56 | 44 |
| LIIIB7 | Myxus capensis + other mugilids | 397 | 56 | 44 |
| LIIIB4 | Epinephelus spp | 46 | 57 | 43 |
| FII A1 | Sardinops sagax | 153669 | 57 | 43 |
| KIIIA10 | Parupeneus spp. | 14694 | 57 | 43 |
| KIIIA1 | Chirodactylus jessicalenorum | 4573 | 57 | 43 |
| KIIIB2A | Cirrhitichthys oxycephalus | 751 | 58 | 42 |
| FIIA7 | Oplegnathus conwayi | 75 | 59 | 41 |
| LII A3 | Aulostomus chinensis | 200 | 59 | 41 |
| FIIIA3 | Paralichthodes algoensis | 94 | 60 | 40 |
| LIIIA11 | Auxis rochii | 18905 | 60 | 40 |
| K III B3A | Gymnocranius cf griseus | 390 | 60 | 40 |
| LIIB7 | Apistus carinatus | 92 | 61 | 39 |
| CMI A1 | Ostraciidae | 593 | 61 | 39 |
| KIIIB3 | Priacanthus hamrur | 1086 | 61 | 39 |
| MII A5 | Pempheridae | 370 | 61 | 39 |
| FIIIA4 | Pomatomus saltatrix | 3810 | 62 | 38 |
| DIIIA3 | Callionymidae | 991 | 63 | 37 |
| FII B1 | Neoscorpis lithophilus | 152 | 63 | 37 |
| DI A1 | Anguilliform | 515 | 64 | 36 |
| HII A3 | ?Choridactylus natalensis | 31 | 65 | 35 |
| LIIIF9 | Bothidae | 274 | 65 | 35 |
| LIIID2 | Epinephelus spp. | 784 | 67 | 33 |
| HII A4 | Suarida undusquamis | 1183 | 67 | 33 |
| EIIIA2 | Oplegnathus robinsoni | 88 | 68 | 32 |
| FIIIA6 | Pomacanthus rhomboides | 260 | 68 | 32 |
| DII A1 | Etrumeus teres | 65917 | 69 | 31 |
| LIIIG4 | Acanthurus triostegus | 4212 | 69 | 31 |
| EIIA2 | Seriola dumerili & S. lalandii | 99 | 70 | 30 |
| LII B5 | Lepidotrigla faurei | 1324 | 70 | 30 |
| KIIIB7 | Calotomus carolinus & Labridae | 1068 | 71 | 29 |
| LIIIE9 | Pseudorhombus elevatus | 241 | 71 | 29 |
| BKIIIA2 | Scarus spp | 5464 | 71 | 29 |
| HI A3 | Fistularia spp | 197 | 73 | 27 |
| LIIIE4 | Antigonia rubescens | 243 | 73 | 27 |
| LIIIA8 | Argyrosomus japonicus | 4311 | 73 | 27 |
| LIIIF3 | Cubiceps pauciradiatus | 159 | 74 | 26 |
| EII A3 | Centroberyx spinosus | 1737 | 74 | 26 |
| LII A7 | Scomber japonicus | 108855 | 75 | 25 |
| LIA4 | Brama brama | 76 | 75 | 25 |
| BDIIIA1 | Engraulus encrasicolus & Encrasicholina punctifer | 19317 | 76 | 24 |
| LIIIA4 | Umbrina canariensis + | 560 | 76 | 24 |
| GIA1 | Anguilliform | 85 | 76 | 24 |
| EIIIB6A | Myripristis berndti | 250 | 80 | 20 |
| FII A4 | Coryphaena hippurus | 307 | 81 | 19 |
| EIIIA9 | Emmelichthys struhsakeri | 941 | 81 | 19 |
| CDI A2 | Scomberesox saurus | 119 | 82 | 18 |
| LII B3 | Aulacocephalus temmincki | 324 | 83 | 17 |
| LI A1 | Caristius spp. | 126 | 83 | 17 |
| KIIIB1 | Pristipomoides sieboldii & Paracaesio sordida | 6492 | 84 | 16 |
| KIIIA9 | Paracaesio xanthura & Caesio cf caerulaurea | 60050 | 84 | 16 |
| LII A6 | Atractoscion aequidens | 3040 | 85 | 15 |
| DIIIA1 | Vinciguerria nimbaria | 510 | 85 | 15 |
| ABHIIIA1 | Parascorpaena mossambica + | 1641 | 85 | 15 |
| EIIIA8 | Centroberyx druzhinini | 91 | 86 | 14 |
| EIIIA1 | Sphyraena jello | 153 | 88 | 12 |
| BHIIA1 | Unknown | 109 | 88 | 12 |
| BKIIIA1 | Carapidae | 1396 | 88 | 12 |
| LIIIE8 | Heniochus acuminatus | 350 | 89 | 11 |
| FII A5 | Centrolophus niger | 1227 | 89 | 11 |
| FIIIA1 | Acanthistius joanae | 793 | 90 | 10 |
| EII A4 | Seriola dumerili & S. rivoliana | 640 | 90 | 10 |
| EIIIB5 | Pristigenys niphonia | 127 | 92 | 8 |
| LIIIA11B | Katsuwonus pelamis | 153 | 92 | 8 |
| FII A8 | Plagiogeneion rubigonosum | 53 | 92 | 8 |
| LIIIE10 | Zebrasoma gemmatum | 177 | 93 | 7 |
| DIIIA4 | Unknown | 1472 | 95 | 5 |
| KIIIB5 | Unknown | 79 | 97 | 3 |
| BLIIIA2 | Scorpaenidae | 649 | 100 | 0 |
7.4 The most common eggs from Durban Bay and offshore
The following two tables summarise the most common eggs, based on total numbers found over the duration of each study.
Table 2: The most common eggs collected in the mouth of Durban Harbour on outgoing spring tides, from May 1990 to December 1994, arranged in order of abundance (number of samples =77).
|
n |
Code |
Species or description |
n |
Code |
Species or description |
|
10531 |
LIIIE3A |
Acanthopagrus vagus |
13 |
DIIIA3 |
Callionymus marleyi |
|
9263 |
GII A1 |
Hilsa kelee |
11 |
FIIIA2A |
Dichistius multifasciatus |
|
5484 |
EIIIB2 |
Pomadasys commersonnii |
11 |
LIIIA5 |
Cociella heemstrai |
|
4727 |
LIIID9 |
Rhabdosargus sarba |
11 |
LIIIC9 |
Pseudorhombus arsius |
|
4333 |
LIIIA7 |
Liza dumerilii and other mugilids |
10 |
LII A2 |
Pempheris schwenkii |
|
3646 |
DI A1 |
Anguilliformes |
8 |
KIIIB7 |
Labridae |
|
1799 |
KIIIB2A |
Cirrhitichthys oxycephalus |
8 |
LIIIF7 |
Unknown |
|
1312 |
LIIIC1 |
Diplodus capensis, blacktail |
7 |
LIIIE1A |
Parapercis sp |
|
1142 |
LIIIF5 |
Secutor ruconius |
7 |
LIIIE3 |
Sciaenidae |
|
986 |
BFIIIA1 |
Stolephorus holodon |
7 |
LII A3 |
Aulostomus chinensis |
|
948 |
KIIIB9 |
Ambassis dussumieri |
6 |
MII A1 |
Dagetichthys marginatus |
|
874 |
EIIIB8 |
Gerres acinaces |
6 |
LII B5 |
Lepidotrigla faurei |
|
853 |
DII A2 |
Thryssa setirostris |
5 |
EIIIA6 |
Trachurus trachurus & Decapterus spp. |
|
637 |
LIIIE11 |
Crenidens crenidens |
5 |
KIIIA1 |
Chirodactylus jessicalenorum |
|
306 |
MIIIA2 |
Thysanophrys celebica |
5 |
LIIIA11 |
Auxis rochei |
|
249 |
LIIIG4 |
Acanthurus triostegus |
5 |
LIIIC3 |
Pseudorhombus sp. |
|
130 |
KIIIB8 |
Halichoeres lapillus + |
4 |
BDIIIA1 |
Engraulis encrasicolus & Encrasicholina punctifer |
|
123 |
LIIID2 |
Epinephelus spp. |
4 |
KIIIA6 |
Brotula multibarbata |
|
118 |
LIIIF2 |
Chaetodon dolosus & C. blackburni |
4 |
LII B9 |
Liza tricuspidens |
|
114 |
LIIIG7 |
Arnoglossus |
4 |
MIIIA5 |
Solea turbynei (bleekeri) |
|
94 |
LIIIE1 |
Parapercis robinsoni |
3 |
BKIIIA2 |
Scarus rubroviolaceus |
|
66 |
MIIIA8 |
Cynoglossus sp |
3 |
EIIIB4 |
Pomadasys olivaceum |
|
65 |
KIIIC1 |
Pseudanthias squamipinnis |
3 |
FIIIA4 |
Pomatomus saltatrix |
|
57 |
KIIIA10 |
Parupeneus spp. |
3 |
HI A2 |
Fistularia petimba |
|
56 |
KIIIA2 |
Anchichoerops natalensis |
3 |
HII A1 |
Tetraodontidae |
|
54 |
DII A1 |
Etrumeus teres |
3 |
KIIIA9 |
Caesio cf caerulaurea |
|
53 |
EIIIB1 |
Upeneus guttatus |
2 |
FI A1 |
Anguilliformes |
|
50 |
HII A4 |
Saurida undusquamis |
2 |
HII A3 |
Choridactylus natalensis |
|
39 |
FII A9 |
Monodactylus falciformis |
2 |
KIIIC3 |
Thalassoma trilobatum |
|
38 |
FIIIA4B |
Sillago cf sihama |
2 |
LI A2 |
Trichiurus lepturus |
|
34 |
LII A5 |
Aeoliscus punctulatus |
2 |
LIIIA4 |
Umbrina canariensis (?+ others) |
|
30 |
LIIIA6 |
Platycephalus indicus |
2 |
MIIIA7 |
Limnichthys nitidus |
|
28 |
LIIIB10 |
Pachymetapon grande |
1 |
CMI A1 |
Tetrosomus concatenates |
|
25 |
MII A2A |
Asseragodes heemstrai |
1 |
KIIIB3 |
Priacanthus hamrur |
|
18 |
FII A1 |
Sardinops sagax |
1 |
FIIIA2 |
Dinoperca petersi |
|
17 |
DIIIA1 |
Phosichthyidae |
1 |
LII A7 |
Scomber japonicus |
17 |
KIIIB9A |
Unknown |
1 |
LII B6 |
Umbrina robinsoni |
16 |
CHII A1 |
Trachinocephalus myops |
1 |
LII B8 |
Scorpaenidae |
16 |
LIIIB9 |
Sarpa salpa |
1 |
LIIIE7 |
Acanthurus mata |
15 |
LIIIC5 |
Pagellus natalensis |
1 |
MI A1 |
Lampridiformes |
14 |
KIIIA4 |
Serranus knysnaensis |
1 |
MIIIA1 |
Rogadius portuguesus |
14 |
KIIIA8 |
Paracaesio xanthura |
1 |
MIIIA4 |
?Apodocreedia vanderhorsti |
14 |
LII B4 |
Diplodus hottentotus |
Table 3: The top 154 most common eggs from Park Rynie samples, collected from 1987 to 2011 inclusive, arranged in order of abundance.
|
n |
Code |
Species or description |
n |
Code |
Species or description |
|
234342 |
FII A1 |
Sardinops sagax |
355 |
LIIIE8 |
Heniochus acuminatus |
|
194601 |
LII A7 |
Scomber japonicus |
346 |
MIIIA1 |
Grammoplites portuguesus |
|
114385 |
DII A1 |
Etrumeus teres & E. whiteheadi |
341 |
FIIA4 |
Coryphaena hippurus |
|
95583 |
LIIIC5 |
Pagellus natalensis |
341 |
LIIB4 |
Diplodus hottentotus |
|
61038 |
KIIIA9 |
Paracaesio xanthura & Caesio caerulaurea |
326 |
LIIIF7 |
Unknown |
|
56310 |
EIIA6 |
Trachurus & Decapterus |
298 |
KIIIB1A |
Paracaesio sordida |
|
43420 |
EIIIB3 |
Pomadasys striatum |
294 |
LIIIE9 |
Pseudorhombus elevatus |
|
30777 |
LIIIC1 |
Diplodus capensis |
291 |
LIIIF9 |
Engyprosopon grandisquama |
|
24988 |
BDIIIA1 |
Engraulis encrasicolus & Encrasicolina punctifer |
274 |
FIIIA6 |
Pomacanthus rhomboides |
|
21688 |
LIIIA11 |
Auxis rochii |
250 |
EIIIB6A |
Myripristis berndti |
|
21593 |
LIIIG7 |
Crossorhombus valdirostratus |
244 |
FIIIA2 |
Dinoperca petersi |
|
16853 |
KIIIA10 |
Parupeneus fraserorum & P. rubescens |
237 |
CDIIIA1 |
Draculo celetus + |
|
12443 |
KIIIB8 |
Halichoeres lapillus + |
232 |
HIA3 |
Fistularia commersonii & F. petimba |
|
9382 |
LIIIE7 |
Acanthurus mata |
229 |
MIIA1 |
Dagetichthys marginatus |
|
8237 |
LIIIA8 |
Argyrosomus japonicus |
223 |
EIIIB6 |
Caranx sem |
|
7947 |
LIIIA5 |
Secutor insidiator |
216 |
MIIA6 |
Cynoglossus lida |
|
7179 |
LIIIB9 |
Sarpa salpa |
211 |
LIIIE5 |
Macroramphosus scolopax |
|
6699 |
KIIIB1 |
Paracaesio sordida & Pristipomoides sieboldii |
208 |
LIIA3 |
Aulostomus chinensis |
|
6652 |
BKIIIA2 |
Scarus rubroviolaceus & S. ghobban |
205 |
CDIA2 |
Scomberesox saurus |
|
6590 |
MIIIA8 |
Cynoglossidae | 204 |
LIIB6 |
Umbrina robinsoni |
|
6235 |
CHII A1 |
Trachinocephalus myops |
193 |
FIIIA2A |
Dichistius multifasciatus |
5933 |
FIIIA4 |
Pomatomus saltatrix |
183 |
EIIIB5 |
Pristigenys niphonia |
4933 |
KIIIA1 |
Chirodactylus jessicalenorum |
181 |
KIIIA2 |
Anchichoerops natalensis |
4929 |
EIIIB4 |
Pomadasys olivaceum |
181 |
MIIIA2 |
Onigocia oligolepis + |
4647 |
KIIIA8 |
Paracaesio xanthura |
179 |
LIIIE10 |
Zebrasoma gemmatum |
4483 |
LIIIG4 |
Acanthurus triostegus |
173 |
LIIA11B |
Katsuwonus pelamis |
4268 |
LIIIF2 |
Chaetodon spp. |
169 |
LIIIF3 |
Cubiceps pauciradiatus |
3386 |
FII A9 |
Monodactylus falciformis |
163 |
LIIIC9 |
Pseudorhombus arseus |
3213 |
LIIA6 |
Atractoscion aequidens |
160 |
FII B1 |
Neoscorpis lithophilus |
2830 |
ABHIIIA1 |
Scorpaenopsis possi & Parascorpaena mossambica |
157 |
EIIIA1 |
Sphyraena jello |
2810 |
LIIIB10 |
Pachymetopon grandis |
145 |
MIIIA5 |
Pagusa nasuta |
2330 |
KIIIC1 |
Pseudanthias fasciatus & P. squamipinnis |
143 |
FIA1 |
Anguilliform |
2112 |
LIIB5 |
Lepidotrigla faurei |
138 |
FIIIA7 |
Sillago cf sihama |
2095 |
EII A3 |
Centroberyx spinosus |
134 |
LIA1 |
Caristius sp |
2062 |
EIIIB1 |
Upeneus guttatus |
131 |
LIIIA6 |
Platycephalus indicus |
1853 |
HIIA4 |
Saurida undosquamis |
130 |
LIIA5 |
Aeoliscus punctulatus |
1812 |
LIIIE1 |
Parapercis robinsoni + |
127 |
LIIB9 |
Myxus capensis & Liza tricuspidens |
1704 |
DIIIA4 |
Callionymidae |
123 |
GIA1 |
Anguilliform |
1586 |
KIIIB7 |
Labridae & Calotomus carolinus |
119 |
BHIIA1 |
Unknown |
1527 |
BKIIIA1 |
Carapidae |
118 |
EIIIA8 |
Centroberyx druzhinini |
1517 |
DIIIA3 |
Callionymus marleyi |
117 |
EIIIA2 |
Oplegnathus robinsoni |
1452 |
LIIID1 |
Lithognathus mormyrus |
110 |
FIIIA2 |
Paralichthodes algoensis |
1426 |
KIIIA6 | Brotula multibarbata | 109 |
EIIA2 |
Seriola dumerili & S. lalandi |
1364 |
FIIA5 |
Centrolophus niger |
108 |
LIIB7 |
Apistus carinatus |
1230 |
KIIIB3 |
Priacanthus hamrur |
107 |
CMIA2 |
Zebrias cf regani |
1220 |
LIIID9 |
Rhabdosargus sarba | 104 |
KIIIB5 |
Unknown |
1217 |
FIIIA1 |
Acanthistius joanae |
89 |
LIIIB4 |
Epinephelus malabaricus & E. andersoni |
| 1125 | LIIIA4 |
Umbrina canariensis |
88 |
FIIA7 |
Oplegnathus conwayi |
1019 |
EIIIA9 |
Emmelichthys struhsakeri |
84 |
LIA4 |
Brama brama |
1008 |
BFIIIA1 |
Stolephorus holodon |
83 |
LIA2 |
Trichiurus lepturus |
953 |
MII A2 |
Sarda orientalis |
81 |
FIIA8 |
Plagiogeneion rubiginosum |
931 |
LIIA2 |
Pempheris schwenkii |
73 |
HIIA3 |
Choridactylus natalensis |
890 |
KIIIB2A |
Cirrhitichthys oxycephalus |
68 |
CHIA1 |
Oxyporhamphus micropterus |
825 |
LIIID2 |
Epinephelus rivulatus + |
68 |
CLIIA2 |
Myctophiform |
786 |
CMIA1 |
Ostraciidae | 64 |
CDIA1 |
Regalecus glesne |
783 |
KIIIA4 |
Serranus knysnaensis |
63 |
GIIA1 |
Hilsa kelee |
775 |
DIIIA1 |
Vinciguerria nimbaria |
60 |
FIIIA9 |
Sillago chondropus |
748 |
LIIIA5 |
Cociella heemstrai |
59 |
LIIB8 |
? Scorpaenidae |
689 |
EIIA4 |
Seriola spp |
48 |
MIA3 |
Diodon holocanthus |
663 |
MIIIA7 |
Limnichthys nitidus |
46 |
LIIIE3A |
Acanthopagrus vagus |
652 |
BLIIIA2 |
Scorpaenidae |
43 |
EII A1 |
Pentaceros capensis |
634 |
DIA1 |
Anguilliforms |
42 |
EIII A3A |
Sphyraena sp |
570 |
LIIIB7 |
Liza dumerilii + |
42 |
HIIA2 |
Scorpaenidae |
523 |
LIIIA11A |
Euthynnus affinis |
40 |
ABKIIIA1 |
Carapidae |
498 |
LIIA6A |
Tripterodon orbis |
39 |
FIIA3 |
Hyperoglyphe antarctica |
466 |
KIIIB2 |
Cyprinocirrhites polyactis |
36 |
LIIIG1 |
Bothidae |
465 |
LIIICiB |
Pachymetopon aeneum |
35 |
KIIIC2 |
Anthiinae (sea goldie) |
456 |
EIIIB4A |
Megalaspis cordyla |
33 |
LIIA4A |
Scomberomorus plurilineatus |
443 |
MIIA5 |
Parapriacanthus ransonneti |
33 |
LIIB1 |
Champsodon capensis |
443 |
MIIIA4 |
?Apodocreedia vanderhorsti |
32 |
LIIIB3 |
Sparidae |
427 |
EIIIA3 |
Carangidae |
31 |
BLIIIA1 |
Helicolenus dactylopterus |
425 |
FIIIA5 |
Chartodon marleyi |
30 |
ABHIIIA2 |
Scorpaenidae |
413 |
EIIIB8 |
Gerres acinaces |
30 |
LIIIC3 |
Pseudorhombus sp. |
408 |
LIIB3 |
Aulacocephalus temminckii |
27 |
LIIID4 |
Cephalopholis sonnerati |
380 |
KIIIC3 |
Thalassoma trilobatum |
26 |
LIIIE3 |
?Argyrosomus thorpei |
365 |
KIIIB3A |
Gymnocranius griseus |
25 |
EIIIA1A |
Kyphosus bigibbus |
359 |
LIIE4 |
Antigonia rubescens |
25 |
LIIIC4 |
Malacanthus brevirostris |
8. Voucher specimens.
Representative samples of all reared larvae and juveniles have been archived, and are deposited in the SAIAB collection at Rhodes University in Grahamstown.
Professor Paul Hebert and his team, particularly Tyler Zemlak and Dirk Steinke, at the University of Guelph, Canada, whose assistance with barcoding of larvae has been a mainstay to identifying larvae, thereby contributing hugely to the value of the dataset.
The MODIS Project and the Active Archive Centre at the Goddard Space Flight Centre, Greenbelt, MD 20771, for the production of MODIS data, and Bertrand Saulquin for providing these data (Plates 3, 9 and 10).
Park Rynie Skiboat Club, for use of their launch facilities at Rocky Bay.
The CSIR, which funded the sample collection from 1987 to 2003, and for permission to use Plates 4-8 in the Introductory Notes.
The Oceanographic Research Institute, Durban, for allowing me to collect eggs from the main tank, and for collaborative work with staff, particularly Dr Pat Garratt.
The South African Institute for Aquatic Biodiversity, Grahamstown, for ichthyological expertise, and for becoming involved in the "Barcode of fishes" initiative.
Mike, Valda and Alan Fraser, for their interest and insights, fish DNA samples, and for assisting with sample collection while I was incapacitated between March and November 2006.
My network of fish collectors, for DNA barcoding of adult fishes, including Knud Sorensen, captain of the trawler Ocean Spray, Tim McClurg and Steven Weerts at CSIR Durban, Rob Broker and Paul Buchel at KZN Wildlife, Sheldon Dudley at Natal Sharks Board, Simon Chater, Sean Fennessy and Bruce Mann at ORI, Andre Bok at I&J in Danger Point, Ken and Larry Hutchings at University of Cape Town and, especially, Rob Cooper at Marine & Coastal Management in Cape Town.
Dr Phil Heemstra and Elaine Heemstra, for ichthyological expertise and many DNA samples.
FC (Billy) Clark, whose experience of the sea and fishing commercially for "74" and other species of linefish in the 1950’s and 60’s, aboard his vessel The Plough, along the KZN and eastern Cape coast, has added interesting anecdotal information to some of the species sheets.
Ahlstrom EH, Butler JL & Sumida BY. 1976. Pelagic stromateoid fishes (Pisces, Perciformes) of the eastern Pacific: kinds, distributions, and early life histories and observations of five of those from the Northwest Atlantic. Bulletin of Marine Science 26: 285-402.
Ahlstrom EH & Counts RC. 1958. Development and distribution of Vincihuerria lucetia and related species in the eastern Pacific. United States Fisheries Bulletin 58: 363-416.
Ahrens R L 1964. A preliminary report on the biology of the seventy- four Polysteganus undulosus (Regan) in Natal waters. Unpublished M.Sc. thesis. University of Natal, Durban: pp 1-77.
Anders AS. 1975. Pilchard and anchovy spawning along the Cape east coast. South African Shipping News and Fishing Industry Review 30: 53-57.
Badenhorst P, Cooper JAG, Crowther J, Gonsalves J, Grobler NA, Illenberger WK, Laubscher WI, Mason TR, Moller JP, Perry JE, Reddering JSV and van der Merwe L. 1989. Survey of September 1987 Natal floods. South African National Scientific Programmes Report 164: 1-135.
Beckley LE. 1986. The ichthyoplankton assemblage of the Algoa Bay nearshore region in relation to coastal zone utilisation by juvenile fish. South African Journal of Zoology 21: 244-252.
Beckley LE and Connell AD. 1996. Early life history of Pomatomus saltatrix off the East coast of South Africa. Marine and Freshwater Research 47: 319-322.
Beckley LE and Hewitson JD. 1994. Distribution and abundance of clupeoid larvae along the east coast of South Africa in 1990/1991. South African Journal of Marine Science 14: 205-212.
Beckley LE and Naidoo AK. 2003. Exploratory trials with light-traps to investigate settlement stage fishes in subtropical, coastal waters off South Africa. South African Journal of Zoology 38: 333-342.
Begg G. 1978. The estuaries of Natal. Natal Town and Regional Planning, 41: 1-657.
Blaber SJ, Cyrus DP and Whitfield AK. 1981. The influence of zooplankton food resources on the morphology of the estuarine clupeid Gilchristella aestuarius (Gilchrist 1914). Environmental Biology of Fishes 6: 351-355.
Brownell CL. 1979. Stages in the early development of 40 marine fishes with pelagic eggs from the Cape of Good Hope. Ichthyological Bulletin of the J.L.B. Smith Institute of Ichthyology. 40: 84p.
Carpenter KE. 2001. Lethrinidae. In: Carpenter KE and Niem VH.(Eds.) FAO species identification guide for fisheries purposes. The living marine resources of the Western Central Pacific. Volume 5. Bony fishes Part 3(Menidae to Pomacanthidae). Rome FOA. pp. 3004-3050.
Carter RA. 1977. The distribution of calanoid copepods in the Agulhas Current system off Natal, South Africa. MSc Thesis, University of Natal, 165pp.
Carter R and D’Aubrey J. 1988. Inorganic nutrients in Natal continental shelf waters. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, pp 132-151.
Carter R and Schleyer M H. 1988. Plankton distributions in Natal coastal waters. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, pp 152-177.
Cohen DM 1986. Family No 88 Gadidae; Family No 89: Merlucciidae; Family No 90: Moridae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes:324-328.
Collette BB. 1986. Family No. 249: Scombridae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 831-838.
Connell AD. 1985. Neuston netting. In: Environmental studies at Richards Bay prior to the discharge of submarine outfalls: 1974-1984. CSIR Durban Marine Research Group Report. Pp.175-177.
Connell AD. 1989. Neuston netting. In: Environmental studies in the Richards Bay Offshore Outfalls Region. Part 2. Surveys made between 1985 and 1988. CSIR Report EMA-C 8984 pp141-144.
Connell AD. 1996. Sea fishes spawning pelagic eggs in the St Lucia estuary. South African Journal of Zoology 31:37-41.
Connell AD 1997. Seasonal trends in sardine spawning at Park Rynie, Kwazulu-Natal south coast. WOSAS – Workshop on Southern African sardine, (eds.) M. Barange & C. van der Lingen. Sea Fisheries Research Institute Benguela Ecology Programme Report 29:29-33.
Connell AD. 1997a. Neuston netting. In: Environmental studies in the Richards Bay Offshore Outfalls Region. Report No. 10. Surveys made during 1996. CSIR Report ECP-97-011, Part 7: 147-154.
Connell AD. 1998. Neuston netting. In: Environmental studies in the Richards Bay Offshore Outfalls Region. Report No. 11. Surveys made during 1997. CSIR Report ENV/ECP/EXT:97-JEC17, Part 7: 147-154.
Connell AD. 2001. Pelagic eggs of marine fishes from Park Rynie KwaZulu-Natal, South Africa: seasonal spawning patterns of the three most common species. African Zoology 36: 197-204.
Connell AD. 2003. Neuston netting. In: Environmental studies in the Richards Bay Offshore Outfalls Region. Report No. 16: Surveys made during 2002. CSIR Report ENV-D-C-2003-003, Part 7: 153-158.
Connell AD. 2010. A 21-year ichthyoplankton collection confirms sardine spawning in KwaZulu-Natal waters., African Journal of Marine Science 32: 331-336.
Connell AD, Heemstra PC and Garratt PA. 1999. Eggs and larvae of the santer Cheimerius nufar from KwaZulu-Natal. South African Journal of Marine Science 21: 41-50.
Cowley PD, Whitfield AK and Bell KNI. 2001. The surfzone ichthyoplankton adjacent to an intermittently open estuary, with evidence of recruitment during marine overwash events. Estuarine Coastal and Shelf Science 52: 339-348.
Cyrus DP, Vivier L and MacKay CF. 2005. Lake St Lucia fish survey- December 2004. Coastal Research Unit of Zululand – Investigational Report No. 118: 1-27.
Delsman HC. 1922. Fish eggs and larvae from the Java Sea. 2. Chirocentrus dorab. Treubia 3: 38-46.
Delsman HC. 1931. Fish eggs and larvae from the Java Sea. 17. The genus Stolephorus. Treubia, 13: 217-243.
Eschmeyer WN. 1986. Family 149: Scorpaenidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: 463-478.
Fahay MP. 1983. Guide to the early life stages of marine fishes occurring in the western North Atlantic Ocean, Cape Hatteras to the southern Scotian Shelf. Journal of the North West Atlantic Fisheries Science 4: 423 p.
Fahay MP & Markle DF 1983. Gadiformes: Development and Relationships. Pp 265-283. In: American Society of Ichthyologists and Herpetologists Special Publ. 1. Allen Press 760 pp.
Flemming B and Hay R. 1988. Sediment distribution and dynamics on the Natal continental shelf. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, pp 47-80.
Fricke R 1986. Family No. 239: Callionymidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 770-774.
Garratt, PA. 1985. The Offshore Linefishery of Natal: II: Reproductive biology of the sparids Chrysoblephus puniceus and Cheimerius nufar. Investigational Report of the Oceanographic Research Institute of South Africa 63: 21 pp.
Garratt, PA. 1991. Spawning behaviour of Cheimerius nufar in captivity. Environmental Biology of Fishes 31: 345-353.
Garratt PA. 1993. Spawning of the river bream, Acanthopagrus berda, in Kosi estuary. South African Journal of Zoology 28: 26-31.
Gilchrist JDF and Thompson WWF 1914. Descriptions of fishes from the coast of Natal (Part IV). Annals of the South African Museum 13: 65-95.
Griffiths MH and Hecht T. 1995. On the life-history of Atractoscion aequidens, a migratory sciaenid off the east coast of southern Africa. Journal of Fish Biology 47: 962-985.
Haedrich RL 1986. Family No. 254: Stromateidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 842-846.
Hardy JD. 2006. Chiasmodontidae: Swallowers. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 162: 1901-1915.
Harris SA and Cyrus DP. 1998. Composition, abundance and seasonality of fish larvae in the mouth of Durban Harbour, Kwa-Zulu Natal, South Africa. South African Journal of Marine Science 21: 19-39
Harrison TH 2003. Biogeography and community structure of fishes in South African estuaries. PhD Thesis, Rhodes University Grahamstown, South Africa. 229 pp.
Hebert PDN, Ratnasingham S and deWaard JR 2003. Barcoding animal life: Cytochrome C oxidase subunit 1 divergencies among closely related species. Proc. R. Soc London Ser. B 270: S596-S599.
Hecht J. 2003. Every species has a ‘barcode’. New Scientist 22March 2003:pg 14.
Heemstra PC 1986. Family No. 234: Mugiloididae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 739-741.
Heemstra PC 1986a. Family No. 229: Champsodontidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 734-735.
Heemstra PC 1986b. Family No. 261: Cynoglossidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 865-868.
Heemstra PC 1986c. Family No. 230: Uranoscopidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 735-736.
Heemstra PC 1986d. Family No. 126: Berycidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 409-410.
Heemstra PC 1986e. Family No. 79: Synodontidae (revised). In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 270-273.
Heemstra PC 1986f. Family No. 208: Caristiidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 636-637.
Heemstra PC 1986g. Family No. 206 Oplegnathidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 632-633.
Heemstra PC 1986h. Family No. 122 Regalecidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: p. 403.
Heemstra PC and Gon O. 1986. Family No. 262: Soleidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 868-874.
Heemstra PC and Heemstra E. 2004. Coastal Fishes of South Africa. SAIAB and NISC, Grahamstown.
Heemstra PC and Randell JE. 1986. Family No. 165: Serranidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 509-537.
Henseley DA. 1986. Family No. 259: Bothidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 854-863.
Heydorn AEF, Bang ND, Pearce AF, Fleming BW, Carter RA, Schleyer MH, Hughes GR, Bass AJ, van der Elst RP. Crawford RJM and Shelton PA 1978. Ecology of the Agulhas current region: an assessment of biological responses to environmental parameters in the south-west Indian Ocean. Transactions of the Royal Society of South Africa 43: 151-190.
Horn MH. 1984. Stromateoidei: development and relarionships pp 620-628. In: American Society of Ichthyologists and Herpetologists Special Publication 1.
Houde ED, Almatar S, Leak JC and Dowd CE. 1986. Ichthyoplankton abundance and diversity in the western Arabian Gulf. Kuwait Bulletin of Marine Science 8: 107-393.
Hunter IT. 1988. Climate and Weather off Natal. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, pp 81-100.
Hutchings L, Beckley LE, Griffiths MH, Roberts MJ, Sundby S and van der Lingen C. 2003. Spawning on the edge: spawning grounds and nursery areas around the southern African coastline. Marine and Freshwater Research 53: 307-318.
Ikeda T and Mito S. 1988. Pelagic fish eggs pp 999-1083. In: Okiyama (ed) 1988.
Johnson RK and Keene MJ. 1986. Family No. 228: Chiasmodontidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 731-734.
Jones DL, Lara MR and Richards WJ. 2006. Labridae: Wrasses. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 157: 1835-1871.
Joubert CSW. 1981. Aspects of the biology of five species of inshore reef fishes on the Natal coast, South Africa. Investigational Report. Oceanographic Research Institute 51: 1-16.
Knapp LW. 1986. Family No. 155: Platycephalidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 482-486.
Lamkin JT. 2006. Nomeidae: Driftfishes. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 197: 2255-2271.
Leis JM. 1977. Development of the eggs and larvae of the slender mola, Ranzania laevis (Pisces, Molidae). Bulletin of Marine Science 27: 448-466.
Leis JM, Carson-Ewart BM and Cato DH. 2002. Sound detection in situ by the larvae of a coral reef damselfish. Marine Ecology Progress Series 232: 259-268.
Leis JM, and Carson-Ewart BM. 2003. Orientation of pelagic larvae of coral reef fishes in the ocean. Marine Ecology Progress Series 252: 239-253.
Leis JM and Rennis DS. 1983. The larvae of Indo-Pacific coral reef fishes. New South Wales University Press Sydney. 269pp.
Leis JM and Trnski T. 1989. The larvae of Indo-Pacific shorefishes. New South Wales University Press Sydney. 371pp.
Leis JM, Trnski T and Beckley 2002. Larval development of Pagellus natalensis and what larval morphology indicates about relationships in the perciform fish family Sparidae. Mar. Freshwater Res. 53: 367-376.
Lutjeharms JRE and Connell AD. 2000. The Natal Pulse and inshore counter-currents off the South African east coast. South African Journal of Science 85: 533-535.
Lutjeharms JRE, Cooper J and Roberts M. 2000. Upwelling at the inshore edge of the Agulhas Current. Continental Shelf Research 20: 737-761.
Lutjeharms JRE and Roberts HR. 1988. The Natal Pulse: an extreme transient of the Agulhas Current. Journal of Geophysical Research 93 (C1): 631-645.
Lyczkowski-Shulz J. 2006. Molidae: Molas, ocean sunfishes. In: Richards WJ (Ed.) Early Stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 215:2457-2465.
Mann BQ. 2007. Reassessment of the seventy-four Polysteganus undulosus stock after a 10-year moratorium. Oceanographic Research Institute, Durban, ORI Unpublished Report 244: pp. 1-22.
Marcus LR. 2006. Caristiidae: Manefish. In: Richards WJ (Ed.) Early Stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 135:1539-1543.
Matarese AC, Kendell AW, Blood DM and Vinter BM. 1989. Laboratory guide to early life history stages of Northeast Pacific fishes. NOAA Technical Report NMFS 80: 649p.
McKay RJ. 1986. Family No. 198: Sillaginidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 615-616.
Merrett NR. 2006. Bathygadidae & Macrouridae. In: Richards WJ (Ed.) Early Stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 43:595-616.
Mito S 1961. Studies on the pelagic fish eggs and hatched larvae found in the adjacent waters of Japan. Records of Oceanographic Works in Japan. Special No. 5. Pp 71-94.
Mito S 1961a. Pelagic fish eggs from Japanese waters – I. Clupeina, Chanina, Stomiatina, Myctophida, Anguillida, Belonida and Syngnathida. Scientific Bulletin, Faculty of Agriculture, Kyushu University 18(3):285-310 plus plates 20-34 (in Japanese, English summary).
Mito S 1961b. Pelagic fish eggs from Japanese waters – II. Lamprida, Zeida, Mugilina, Scombrina, Carangina and Stromateina. Scientific Bulletin, Faculty of Agriculture, Kyushu University 18(4):451-466 plus plates 39-43 (in Japanese, English summary).
Mito S 1962. Pelagic fish eggs from Japanese waters – V. Callionymina and Ophidiina. Scientific Bulletin, Faculty of Agriculture, Kyushu University 19(3):377-380 plus plates 11-12 (in Japanese, English summary).
Mito S 1963. Pelagic fish eggs from Japanese waters – III. Percidae Japanese Journal of Ichthyology 11(1-2):39-64 plus plates 1-18 (in Japanese, English summary).
Mito S 1963b. Pelagic fish eggs from Japanese waters – IX. Echeneida and Pleuronectida. Japanese Journal of Ichthyology 11(3-6):81-102 plus plates 29-41 (in Japanese, English summary).
Mito S 1963c. Pelagic fish eggs from Japanese waters –X. Gadida and Lophiida. Japanese Journal of Ichthyology 11(3-6):103-113 plus plates 42-45 (in Japanese, English summary).
Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW and Richardson SL. 1984. Ontogeny and systematics of fishes. Special Publication 1, American Society of Ichthyology and Herpetology. Allen Press 760p.
Nakamura I. 1986. Family No. 248: Trichiuridae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 829-830.
Okiyama M. (ed) 1988. An atlas of the early stage fishes in Japan. Tokai University Press, Tokyo. 1154pp. (in Japanese).
Olney JE. 2006. Lophotidae: Crestfish & unicornfish. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 82: 999-1003.
Olney JE and Richards WJ. 2006. Trachipteridae: Dealfishes and Ribbonfishes. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 86: 1019-1026.
Pearce AF. 1977. The shelf circulation off the east coast of South Africa. CSIR Research Report 361: 1-220.
Pearce AF. 1978. Seasonal variations of temperature and salinity on the northern Natal continental shelf. South African Geographical Journal 60: 135-143.
Pearce AF, Schumann EH and Lundie GSH. 1979. Features of the shelf circulation off the Natal coast. South African Journal of Science 74: 328-331.
Preston-Whyte RA. 1969. Sea breeze studies in Natal. South African Geographical Journal 51: 38-49.
Randall JE. 1961. A contribution to the biology of the convict surgeonfish of the Hawaiian Islands, Acanthurus triostegus sandvicensis. Pacific Science 15: 215-272.
Randall JE. 1986. Family No. 220: Labridae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 683-706.
Richards WJ. 2006. Scombridae: Mackerels and Tuna. In: Richards WJ (Ed.) Early life stages of Atlantic Fishes: An Identification Guide for the Western Central North Atlantic. Chapter 191: 2187-2227.
Roberts MJ, Zemlak T and Connell AD. 2011. Cyclonic eddies reveal Oegopsida squid egg balloon masses in the Agulhas Current, South Africa. African Journal of Marine Science 33: 239-246.
Schumann EH. 1988. Physical Oceanography off Natal. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, pp 101-130.
Senou H. 2001. Sphyraenidae. In: Carpenter KE and Niem VH.(Eds.) FAO species identification guide for fisheries purposes. The living marine resources of the Western Central Pacific. Volume 6. Bony fishes Part 3(Labridae to Latimeriidae). Rome FOA. pp. 3685-3697.
Shao KT., Yang RS., Chen KC and Lee YS. 2001. An identification guide of marine fish eggs from Taiwan. Institute of Zoology Academia Sinica 176p.
Smith MM. 1986. Family No. 207: Bramidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 633-636.
Smith MM. 1986a. Family No. 185: Lethrinidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 595-600.
Smith MM and Heemstra PC. 1986. Smiths’ Sea fishes. Macmillan, Johannesburg.
Smith MM and Smith JLB. 1986. Family No. 183: Sparidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 580-594.
Smith MM and Smith JLB. 1986a. Family No. 222: Mugilidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 714-720.
Smith-Vaniz WF. 1986. Family No. 210: Carangidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 638-661.
Strydom NA and d'Hotman BD. 2005. Estuary-dependence of larval fishes in a non-estuary associated South African surf zone: evidence of continuity of surf assemblages. Estuarine, Coastal and Shelf Science 63: 101-108.
Thompson EF, Strydom NA and Hecht T. 2007. Larval development of Dagetichthys marginatus (Soleidae), obtained from hormone-induced spawning under artificially reared conditions. Scientia Marina 71: 421-428.
Tighe KA and Keene MJ 1984. Zeiformes: development and relationships. Pp393-398. In: ASIH Special Publication 1. (see Moser et al 1984).
Uchida K, Imia S, Mito S, Fujita S, Ueno M, Shojima Y, Semta T, Tahuka M and Dôtu Y. 1958. Studies on the eggs, larvae and juvenile of Japanese fishes. Series 1. Second Laboratory of Fisheries Biology, Fisheries Department, Faculty of Agriculture. Kyushu University pp 1-89 plus 86 plates (in Japanese).
Vachon J, Desoutter M and Chapleau F. 2005. Solea bleekeri Boulenger, 1898, a junior synonym of Pagusa nasuta (Pallas, 1814), with the recognition and redescription of Solea turbynei Gilchrist, 1904 (Pleuronectiformes, Soleidae). Cybium 29: 314-319.
Van der Elst RP. 1981. A guide to the common sea fishes of Southern Africa. Struik, Cape Town. Pp 1-367.
Van der Elst RP. 1988. Shelf Ichthyofauna of Natal. In: Coastal Ocean Studies off Natal, South Africa. E.H. Schumann (ed.) Berlin. Springer Verlag, Chapter 9: pp 209-225.
Weerts SP. 2002. Habitat utilisation by juvenile fishes in Mhlathuze estuary and Richards Bay harbour. MSc thesis, University of Zululand. Pp 1-211.
Whitehead PJP and Wongratana T. 1986. Family No. 55: Engraulidae. In: Smith & Heemstra (Eds.) Smiths’ Sea Fishes: pp. 204-207.
Whitfield AK. 1990. Life-history styles of fishes in South African estuaries. Environmental Biology of Fishes 28: 295-308.
Whitfield AK. 1994. An estuary-association classification for the fishes of southern Africa. South African Journal of Science 90: 411-419.
Wongratana T, Munroe TA and Nizinski MS. 1999. Engraulidae. In: Carpenter KE and Niem VH. (Eds.) FAO species identification guide for fisheries purposes. The living marine resources of the Western Central Pacific. Volume 3. Batoid fishes, chimaeras and bony fishes Part 1(Elopidae to Linophrynidae). Rome FOA. pp. 1698-1753.
Notes on fish eggs, and the species data sheets.
The age of eggs in the samples.
There are 5 major types:-
i) eggs >2mm diameter, which can take 8-10 days to hatch, depending on species, lapsed incubation time when collected, and ambient temperatures
ii) eggs of 1.5-2mm which might take 48-96 hours to hatch
iii) eggs ca 1-1.5mm which are usually 20-24 hours into incubation when examined. They were spawned the evening before collection, and will usually hatch by the next evening ie ca 48 hours (or on the evening of collection if spawned two evenings before collection)
iv) small eggs around 650-800µm, spawned the evening before, and hatching in the bucket if left too long. Incubation time 24-28 hours
v) fresh eggs, still in the blastula stage. These are the early morning spawners, if collected during the day. In the Park Rynie area, the species known to be in this group are mostly sparids, including Diplodus sargus (LIIIC1), Chrysoblephus puniceus (LIIID5), Cheimerius nufar (LIIID7) and Pagellus natalensis, but also Cynoglossus (MIIIA8) and Arnoglossus (LIIIG7), and MIIIA1 and MIIIA2, among others.
Factors affecting egg density.
Apart from the seasonal factors controlling spawning in individual species, and day to day factors such as sudden water temperature changes, the behaviour of the fish themselves also affect the number of eggs collected. Solitary species, such as Synodontidae, are often seen in pairs, but dispersed, and this is reflected in their eggs seldom being found in high numbers in samples. At the other extreme, the highest individual species abundance of eggs were invariably the shoaling species, such as Sardinops, Etrumeus and Scomber, followed by the aggregating species such as Pomadasys striatum, P. olivaceum, and the sparids Diplodus sargus and Pagellus natalensis (see Table 3). These factors should be kept in mind when looking at the data sheets. Note also that by deliberately choosing to sample in the evening at Durban Harbour mouth, any early morning spawners would have been severely under-sampled, since 80-90% of eggs were fresh, and anything older would have been spawned the previous evening ( or early morning), diluted outside the harbour on the outgoing tide, and washed back into the harbour on the next incoming tide.
There is also a collection bias created by the incubation time of the eggs of different species. Thus comparisons of catch-rate should be made with caution unless the eggs have a similar incubation time.
As noted in Methods, I always used reflected light on the dissecting microscope, as I found it preferable for seeing all shades of yellow pigment.
Segmentation within the yolk, when present, is variable. In some species it is evenly distributed through the yolk in small interlinking semicircles, like fine cob-webbing. In others, it can be more symmetrical, in one or two rings near the outer edge of the yolk. In some, for example Trachurus, it is bold and strongly refractory; in others it is more subtle, and requires a light and dark edge under the egg, with reflected light, to see it clearly. I find a small piece of white paper, slid under the watchglass, sitting on a black base, the most convenient.
Yolk surface is another character used, and should not be confused with yolk segmentation. The yolk surface can be smooth or evenly rough, like sandpaper, or the bumps may be a little more separated, variously described here as moon-scaped or goose-pimpled. When examining eggs, be sure they are floating and not touching the bottom of the vial. That way those with oil globules orientate correctly, as seem in the species sheets’ photographs. Some eggs become more dense, and sink to the bottom of the bowl as they develop. Notes on these will be found in the individual sheets.
The oil globule, when present, is always clear (i.e. unsegmented), but it can vary in colour, from colourless, through many shades of amber, to red. The paler shades of amber and pink are best seen on a white background. When there is pigment on the surface of the oil globule, as a rule the yellow pigment is dorsal, and the black pigment ventral, when viewed in the normal resting position, with the oil globule upward.
Size versus age in fish larvae.
For consistency, I have described the larvae by age. But the preference is for size (eg flexion is completed by 6.7mm SL). Thus all larvae and juveniles are measured. So while the discussion will give an event occurring at 15 days, reference to the image will immediately inform the reader of the size of the fish at that point. All measurements are of the live animal unless otherwise noted, and all measurements are notochord length (NL) in preflexion and flexion larvae, and standard length (SL) for postflexion.
Abbreviations and definitions. (see Leis & Rennis for further definitions).
BOLD – Barcode of Life Database
DHM – Durban Harbour Mouth
flexion – a brief stage in larval development, when the posterior tip of the notochord bends upwards, and the caudal (tail) fin takes on the adult appearance
gut length as a percentage of NL – the gut measurement in this context is taken from the same point as the NL, ie the horizontal distance from snout to hind edge of anus.
inshore – a sample collected at sea, about 0.5km offshore, over about 15m water depth.
KZN – KwaZulu-Natal, previously known as Natal, the east coast province of South Africa (see Figure 1)
MPA – Marine Protected Area
neuromasts – small sensory organs, comprising a small raised cone with a sensory filament at the tip. Most newly hatched larvae appear to have about 10 scattered over the head and body. Location appears to have no taxonomic value.
NL - notochord length (in mm), from snout to notochord tip, in preflexion and flexion larvae (unless otherwise noted, all measurements are of live specimens).
offshore – a surface sample collected at sea, about 5km from shore, over 40-50m of water depth.
ORI – Oceanographic Research Institute, Durban
PC - post-collection, referring to egg or larval age, with collection set at midday, in offshore samples, unless otherwise noted.
PH – post-hatch (unless otherwise noted, all larval age data is PH)
PVS- perivitelline space (see Figure 13)
 |
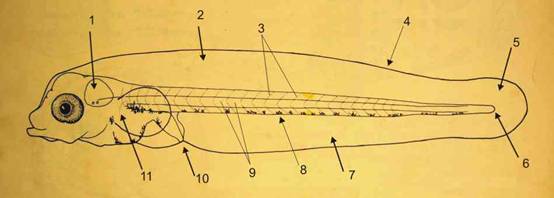 |
Figure 13 Egg: 1: pectoral fin buds, 2: chorion, 3: myomeres and myotomes on the developing embryo, 4: tail wrapped around the yolk; 5: narrow perivitelline space; 6: yolk with stellate black pigment spots; 7: unpigmented eyes; 8: otic capsule. Larva. 1: otic capsule: 2: dorsal finfold; 3: myomeres; 4: serrated finfold edge; 5: caudal finfold; 6: notochord tip; 7: ventral finfold; 8: black pigment ventrally along notochord; 9: myotomes; 10: anus; 11: pectoral fin base. Drawings from Brownell 1979. |
SL - standard length, for postflexion juveniles, from snout to the vertical posterior margin of the hypural plate (tail base, excluding caudal rays).
spiny finfold edges-The edge of the dorsal and ventral finfolds sometimes have small spines embedded, giving a serrated appearance. In most cases it starts in the region of a vertical line through the anus, and ends in the taper towards the caudal finfold. In callionymids it is confined further back, in the unpigmented finfold, and extends into and including the caudal finfold. I have seen it on callionymids, mugiloidids, labrids, acanthurids, some pleuronectiformes such as Arnoglossus, and if Mito’s diagnosis is correct, some lophiids. See sheet KIIIB8, for a list of wrasse genera which have spiny finfold edges. It is usually confined to the 2-5 day larva.
tail – posterior to a vertical line through the posterior edge of the anus
trunk – the body between the head and a vertical line through the posterior edge of the anus
Explanation of the coding system used to categorize the eggs.
A. Eggs in clusters
B. Eggs oval, oblong, or spindle-shaped. Note this does not include asymmetrical eggs, such as KIIIA6, or eggs with flat spots, such as EIIA1
C. Eggs with a sculptured, spiky, nippled or reticulated chorion.
D. Segmented yolk, no oil globule.
E. " " , one oil globule, in anterior (bow) position in the yolksac larva.
F. " " , one oil globule in posterior (stern) position in yolksac larva.
G. " " , multiple oil globules.
H. Clear yolk, no oil globule.
K. " " , one oil globule in anterior (bow) position in yolksac larva.
L. " " , one oil globule in posterior (stern) position in yolksac larva.
M. " " , multiple oil globules.
Size I > 1.5mm diameter
II 1.0 – 1.5mm
III < 1.0mm (=1000µm)
D = Etrumeus, Thryssa, some callionymids
E = carangids, sphyraenids, gerrids, oplegnathids, mullids, haemulids
F = sardine, Coryphaena, stromataeids, sillagids, Acanthistius
G = eels, Gilchristella, Hilsa,
H = fistulariids, some puffers, scorpaenids and lizardfishes
K = anthiinae, some lutjanids, lethrinids, labrids, cheilodactylids, ambassids, mullids
L = sparids, jawfishes, scorpaenids (most), epinephilids, mugilids, sciaenids, scombrids, acanthurids, pomacanthids, chaetodontids, leiognathids
M = some pleuronectiformes and puffers, Sarda.
By applying this simple key, one can quickly position an egg and early larva, and reduce the search options to a small cluster of species pages, where egg size spans that of the unknown species. Conversely, one can quickly decipher that ABHIIIA1 is an egg originally laid in clusters, oval in shape, with a clear yolk and no oil globule, measuring <1mm. Some species will span two size ranges, so it is necessary to check species sheets either side of the apparent range.
Table 4: The species list (alphabetical, by code):
Code |
Order/Family |
|
Size range in µm |
Oil globule |
| ABH III A1 | Scorpaenidae | Scorpaenopsis possi & Parascorpaena mossambica | 870-1000 x 650-870 |
--- |
| ABH III A2 | Scorpaenidae | Unknown | 890-940 x 870-920 |
--- |
| ABK III A1 | Carapidae | Unknown | 1200 x 780 |
35 |
| BD III A1 | Engraulidae | Engraulis encrasicolus & Encrasicholina punctifer | 1160-1310 x 530-605 |
--- |
| BF III A1 | Engraulidae | Stolephorus holodon | 1295-1455 x 680-700 |
120 |
| BH II A1 | Unknown | Unknown | 840-1100 x 790-960 |
--- |
| BH III A1 | Scorpaenidae | Unknown | 1055 x 910 |
--- |
| BK III A1 | Carapidae | Unknown | 960-1000 x 790-820 |
170 |
| BK III A2 | Scaridae | Scarus rubroviolaceus & S. ghobban | 1455 x 500 |
120 |
| BL I A1 | Unknown | Unknown | 2180 x 2060 |
120 |
| BL III A1 | Scorpaenidae | Helicolenus dactylopterus | 915-1060 x 770-840 |
170-190 |
| BL III A2 | Scorpaenidae | Scorpaenodes ? kelloggi | 720-740 x 625-690 |
120-145 |
| BL III A3 | Ogcocephalidae | Halieutaea fitzsimonsi | 840 | 190 |
| CD I A1 | Regalecidae | Regalecus glesne | 2740-2950 |
--- |
| CD I A2 | Beloniformes | Scomberesox saurus | 2280-2920 |
--- |
| CD III A1 | Callionymidae | Draculo celetus + | 620-760 |
--- |
| CH I A1 | Hemiramphidae | Oxyporhamphus micropterus | 1870-2110 |
--- |
| CH I A1A | Exocoetidae | Unknown | 2100-2200 |
--- |
| CH I A2 | Exocoetidae | Cheilopogon nigricans | 1990-2200 |
--- |
| CH II A1 | Synodontidae | Trachinocephalus myops & Synodus indicus | 1070-1140 |
--- |
| CL II A1 | Bothidae | Unknown | 1030-1300 |
140-150 |
| CL II A2 | Myctophidae | Inknown | 960-1125 |
190-205 |
| CM I A1 | Ostraciidae | Tetrosomus concatenatus & Lactoria fornasini | 1800-1900 |
multiple |
| CM I A2 | Soleidae | Zebrias cf regani | 1510-1680 |
multiple |
| CM I A3 | Uranoscopidae | Uranoscopus archionema | 1650-1900 |
multiple |
| CM II A1 | Macrouridae | Caelorinchus or Coryphaenoides sp. | 1225 |
multiple |
| D I A1 | Anguilliformes | Gymnothorax spp. + | 2330-4020 |
--- |
| D II A1 | Clupeidae | Etrumeus teres & E whiteheadi | 1160-1320 |
--- |
| D II A2 | Engraulidae | Thryssa vitrirostris | 985-1060 |
--- |
| D III A1 | Phosichthyidae | Vinciguerria nimbaria + | 625-720 |
--- |
| D III A3 | Callionymidae | Several callionymids but ID uncertain | 680-740 |
--- |
| D III A4 | Lophiiformes | Unknown but may be callionymid | 700-840 |
--- |
| E I A1 | Carangidae | Lichia amia | 1800-1920 |
550-600 |
| E I A1A | Polyprionidae | Polyprion americanus | 2090-2110 |
530 |
| E II A1 | Pentacerotidae | Pentaceros capensis | 1370-1420 |
310-340 |
| E II A2 | Carangidae | Seriola dumerili & S. lalandi | 1200-1450 |
240-360 |
| E II A3 | Berycidae | Centroberyx spinosus | 1200-1250 |
240-265 |
| E II A4 | Carangidae | Seriola dumerili & S. rivoliana | 1000-1075 |
260-270 |
| E III A1 | Sphyraenidae | Sphyraena jello | 910-980 |
240-310 |
| E III A1A | Kyphosidae | Kyphosus bigibbus | 910-985 |
220-250 |
| E III A2 | Oplegnathidae | Oplegnathus robinsoni | 910-940 |
190 |
| E III A2A | Sphyraenidae | Sphyraena obtusata | 940-1010 |
240-265 |
| E III A3 | Carangidae | Unknown | 865-890 |
200-220 |
| E III A3A | Sphyraenidae | Sphyraena sp. | 840-890 |
215-240 |
| E III A4 | Haemulidae | Plectorhynchus chubbi | 940-960 |
215 |
| E III A4A | Carangidae | Carangoides fulvoguttatus | 910-940 |
215 |
| E III A5 | Carangidae | Trachurus trachurus & Decapterus russelli | 850-900 |
210-220 |
| E III A5B | Haemulidae | Plectorhinchus flavomaculatus | 910 |
240 |
| E III A6 | Carangidae | Trachurus trachurus & Decapterus spp. | 745-890 |
205-230 |
| E III A6A | Carangidae | Elagatis bipinnulata | 890 |
240 |
| E III A7 | Carangidae | Scomberoides tol | 815-840 |
290-360 |
| E III A7A | Unknown | Unknown | 815 |
170 |
| E III A7B | Trichonotidae | Trichonotus sp. n. | 790-840 |
190 |
| E III A8 | Berycidae | Centroberyx druzhinini | 960-1030 |
190-220 |
| E III A9 | Emmelichthyidae | Emmelichthys struhsakeri | 745-820 |
170-190 |
| E III B1 | Mullidae | Upeneus guttatus | 720-790 |
170-180 |
| E III B2 | Haemulidae | Pomadasys commersonnii | 800-840 |
190-220 |
| E III B3 | Haemulidae | Pomadasys striatum | 750-820 |
170-190 |
| E III B3A | Kuhliidae | Kuhlia mugil | 850 |
230 |
| E III B4 | Haemulidae | Pomadasys olivaceum | 750-790 |
170-205 |
| E III B4A | Carangidae | Megalaspis cordyla | 800 |
220 |
| E III B5 | Priacanthidae | Pristigenys niphonia | 760 |
175 |
| E III B6 | Carangidae | Caranx sem (=C. heberi) | 760 |
210 |
| E III B6A | Holocentridae | Myripristis berndti | 700 |
190 |
| E III B8 | Gerreidae | Gerres acinaces | 670-770 |
170-220 |
| F I A1 | Anguilliformes | ? Ophichthidae | 1510-1780 |
240-290 |
| F I A1 | Anguilliformes | Unknown | 2500-3410 |
240-190 |
| F I A2 | Melanostomiidae | Opostomias micripnus | 2080-2880 |
265-290 |
| F I A3 | Malacosteidae | Malacosteus niger | 2400-2450 |
265-280 |
| F I A4 | Echeneidae | Remora australis | 2210 |
410 |
| F II A1 | Clupeidae | Sardinops sagax | 1320-1610 |
145-170 |
| F II A3 | Stromataeidae | Hyperoglyphe antarctica | 1250-1345 |
360-410 |
| F II A3A | Trachichthyidae | Gephyroberyx darwinii | 1200 |
240 |
| F II A4 | Coryphaenidae | Coryphaena hippurus | 1320-1560 |
265-340 |
| F II A5 | Stromataeidae | Centrolophus niger | 1200-1345 |
360-410 |
| F II A6 | Nomeidae | Psenes pellucidus | 1225 |
310 |
| F II A7 | Oplegnathidae | Oplegnathus conwayi | 1010-1150 |
240-265 |
| F II A8 | Emmelichthyidae | Plagiogeneion rubiginosum | 1030-1225 |
240-275 |
| F II A8A | Tetragonuridae | Tetragonurus atlanticus | 1150 |
240-265 |
| F II A9 | Monodactylidae | Monodactylus falciformis | 1010-1065 |
240-270 |
| F II B1 | Scorpididae | Neoscorpis lithophilus | 940-1060 |
220-290 |
| F III A1 | Serranidae | Acanthistius joanae | 940-985 |
190-240 |
| F III A2 | Dinopercidae | Dinoperca petersi | 890-960 |
190-220 |
| F III A2A | Dichistiidae | Dichistius multifasciatus | 865-910 |
220-250 |
| F III A2B | Monodactylidae | Monodactylus argenteus | 910-1010 |
240-265 |
| F III A3 | Paralichthodidae | Paralichthodes algoensis | 960-1010 |
180-220 |
| F III A4 | Pomatomidae | Pomatomus saltatrix | 840-960 |
220-265 |
| F III A4B | Sillaginidae | Sillago cf silama | 790-865 |
190-215 |
| F III A5 | Chaetodontidae | Chaetodon marleyi | 790-865 |
190-215 |
| F III A6 | Pomacanthidae | Pomacanthus rhomboides | 790-890 |
190-225 |
| F III A7 | Sillaginidae | Sillago cf sihama | 790-865 |
190-215 |
| F III A9 | Sillaginidae | Sillago chondropus | 745-790 |
190-215 |
| G I A1 | Anguilliformes | Unknown | 1970-3490 |
multiple |
| G I A2 | Monocanthidae | Monocanthus japonicus | 2090-2280 |
multiple |
| G II A1 | Clupeidae | Hilsa kelee | 1080-1175 |
multiple |
| G III A1 | Clupeidae | Gilchristella aestuaria | 865-890 |
multiple |
| H I A1 | Exocoetidae | Exocoetus monocirrhus | 2500-3050 |
--- |
| H I A2 | Trachipteridae | Zu cristatus | 2065-2185 |
--- |
| H I A2A | Lampridae | Lampris cf guttatus | 2350 |
--- |
| H I A3 | Fistulariidae | Fistularia petimba & F. commersonii | 1530-2060 |
--- |
| H II A1 | Pegasidae | Eurypegasus draconis | 1200-1345 |
--- |
| H II A2 | Scorpaenidae | Unknown | 1105-1345 |
--- |
| H II A3 | Scorpaenidae | Choridactylus natalensis + | 1105-1320 |
--- |
| H II A3A | Scorpaenidae | Cocotropus monacanthus | 1000-1320 |
--- |
| H II A4 | Synodontidae | Saurida undosquamis | 1020-1130 |
--- |
| K III A1 | Cheilodactylidae | Chirodactylus jessicalenorum | 910-1030 |
220-260 |
| K III A2 | Labridae | Anchichoerops natalensis | 840-950 |
190-220 |
| K III A4 | Serranidae | Serranus knysnaensis | 860-960 |
145 |
| K III A6 | Ophidiidae | Brotula multibarbata + | 730-1080 |
155-200 |
| K III A8 | Lutjanidae | Paracaesio xanthura | 820-910 |
145-170 |
| K III A9 | Caesionidae | Caesio xanthalytos | 840-910 |
145-190 |
| K III A10 | Mullidae | Parupeneus fraserorum & P. rubescens | 745-890 |
155-190 |
| K III B1 | Lutjanidae | Pristipomoides sieboldii & Paracaesio sordida | 860-910 |
110-170 |
| K III B1A | Lutjanidae | Paracaesio sordida | ||
| K III B2 | Cirrhitidae | Cyprinocirrhites polyactis | 720-840 |
145-170 |
| K III B2A | Cirrhitidae | Cirrhitichthys oxycephalus | 700-790 |
95-110 |
| K III B3 | Priacanthidae | Priacanthus hamrur | 720-770 |
170-180 |
| K III B3A | Lethrinidae | Gymnocranius cf griseus | 625-720 |
145-190 |
| K III B4 | Lethrinidae | Lethrinus rubrioperculatus | 625-720 |
145-190 |
| K III B4A | Caesionidae | Dipterygonotus balteatus | 750 |
145-190 |
| K III B5 | Unknown | Unknown | 720-770 |
145-185 |
| K III B6 | Unknown | Unknown | 700 |
120 |
| K III B7 | Labridae | Unknown | 650-700 |
110-120 |
| K III B8 | Labridae | Halichoeres lapillus + other wrasse | 550-670 |
120 |
| K III B9 | Ambassidae | Ambassis dussumieri | 620-680 |
170-190 |
| K III B9A | Unknown | Unknown | 600-670 |
145-215 |
| K III B9B | Ambassidae | Ambassis ambassis | 625 |
190 |
| K III B9C | Ambassidae | Ambassis natalensis | 600-670 |
190 |
| K III C1 | Serranidae | Pseudanthias gibbosus & P. squamipinnis | 630-690 |
130-150 |
| K III C1A | Serranidae | Pseudanthias connelli | 670 |
120 |
| K III C2 | Serranidae | Anthiinae | 650 |
50 |
| K III C3 | Labridae | Thalassoma trilobatum and other wrasse | 550-625 |
120-145 |
| L I A1 | Caristiidae | Caristius spp. incl. groenlandicus | 1800-1970 |
530-650 |
| L I A2 | Trichiuridae | Trichiurus lepturus | 1620-1825 |
385-455 |
| L I A3 | Zeidae | Zeus faber | 1800-2185 |
310-360 |
| L I A3A | Peristediidae | Satyrichthys adeni | 1620-1990 |
145-170 |
| L I A4 | Bramidae | Brama brama | 1560-1700 |
340-410 |
| L I A5 | Veliferidae | Velifer hypselopterus | 1610-1870 |
145-190 |
| L I A6 | Echeneidae | Remora brachyptera | 1585 |
340 |
| L II A1 | Triacanthodidae | Paratriacanthodes retrospinis | 1200-1415 |
205-220 |
| L II A1A | Bramidae | Taratichthys longipinnis | 1345-1440 |
350-360 |
| L II A2 | Pempheridae | Pempheris schwenkii | 1285-1460 |
270 |
| L II A3 | Aulostomidae | Aulostomus chinensis | 1270-1440 |
220-260 |
| L II A4 | Triglidae | Trigloporus lastoviza africanus | 1295-1320 |
230-325 |
| L II A4A | Scombridae | Scomberomorus plurilineatus | 1250-1320 |
385-410 |
| L II A4B | Luvaridae | Luvarus imperialis | 1300 |
265 |
| L II A5 | Centriscidae | Aeoliscus punctulatus | 1150-1320 |
220-265 |
| L II A5A | Tetrarogidae | Ablabys binotatus | 1300 |
145-215 |
| L II A6 | Sciaenidae | Atractoscion aequidens | 1125-1250 |
280-320 |
| L II A6A | Ephippidae | Tripterodon orbis | 1225-1345 |
240-310 |
| L II A7 | Scombridae | Scomber japonicus | 960-1225 |
240-300 |
| L II A8 | Chiasmodontidae | Chiasmodon niger | 1150-1345 |
310-340 |
| L II A9 | Macroramphosidae | Unknown | 1130-1200 |
240 |
| L II B1 | Champsodontidae | Champsodon capensis | 985-1060 |
170-200 |
| L II B3 | Grammistidae | Aulacocephalus temmincki | 1090-1200 |
170-205 |
| L II B4 | Sparidae | Diplodus hottentotus | 960-1060 |
190-220 |
| L II B5 | Triglidae | Lepidotrigla faurei | 920-1320 |
230-250 |
| L II B6 | Sciaenidae | Unbrina robinsoni | 1000-1060 |
220-250 |
| L II B7 | Scorpaenidae | Apistus carinatus | 910-1100 |
190-220 |
| L II B8 | Scorpaenidae | Unknown | 985-1080 |
200-220 |
| L II B9 | Mugilidae | Liza tricuspidens & Myxus capensis | 985-1060 |
340-360 |
| L II B10 | Unknown | Unknown | 1010 |
240 |
| L II C1 | Bramidae | Unknown | 1080 |
220 |
| L III A1 | Ophidiidae | Hoplobrotula gnathopus | 960-1010 |
220 |
| L III A4 | Sciaenidae | Umbrina canariensis (?+ others ) | 910-1010 |
220-240 |
| L III A5 | Platycephalidae | Cociella heemstrai | 910-985 |
190-240 |
| L III A6 | Platycephalidae | Platycephalus indicus | 940-1030 |
215-265 |
| L III A7 | Mugilidae | Liza dumerilii + other mugilids | 865-980 |
290-350 |
| L III A8 | Sciaenidae | Argyrosomus japonicus | 865-985 |
240-265 |
| L III A9 | Unknown | Unknown | 920 |
220 |
| L III A10 | Dactylopteridae | Dactyloptena peterseni | 960-1010 |
260 |
| L III A11 | Scombridae | Auxis rochei | 865-960 |
220-265 |
| L III A11A | Scombridae | Euthynnus affinis | 865-960 |
220-240 |
| L III A11B | Scombridae | Katsuwonus pelamis | 950-1050 |
240-265 |
| L III B1 | Sparidae | Polysteganus undulosus | 960 |
190 |
| L III B2 | Unknown | Unknown | 950 |
190 |
| L III B3 | Unknown | .Unknown | 960 |
170 |
| L III B4 | Serranidae | Epinephelus malabaricus & E. andersoni | 840-950 |
170-200 |
| L III B7 | Mugilidae | Myxus capensis + other mugilids | 800-920 |
325-370 |
| L III B9 | Sparidae | Sarpa salpa | 875-985 |
190-215 |
| L III B10 | Sparidae | Pachymetopon grande + other sparids | 910-985 |
190-200 |
| L III C1 | Sparidae | Diplodus capensis + other sparids | 890-940 |
190-200 |
| L III C1B | Sparidae | Pachymetopon aeneum | 890-910 |
190 |
| L III C1C | Sparidae | Sparodon durbanensis | 890-910 |
190 |
| L III C2 | Unknown | Unknown | 940-985 |
170-220 |
| L III C3 | Paralichthyidae | ?Pseudorhombus sp | 840-865 |
120-145 |
| L III C4 | Malacanthidae | Malacanthus brevirostris | 890-985 |
170-190 |
| L III C5 | Sparidae | Pagellus natalensis | 790-865 |
170-190 |
| L III C9 | Paralichthyidae | Pseudorhombus arsius | 840-940 |
120-170 |
| L III C9C | Bothidae | Chascanopsetta lugubris | 915 |
145 |
| L III D1 | Sparidae | Lithognathus mormyrus | 910-940 |
170-190 |
| L III D2 | Serranidae | Epinephelus poecilonotus & E. rivulatus | 815-910 |
180-190 |
| L III D4 | Serranidae | Cephalopholis sonnerati | 815-865 |
170-190 |
| L III D5 | Sparidae | Chrysoblephus puniceus | 815-865 |
180 |
| L III D6 | Sparidae | Rhabdosargus holubi + | 850 |
180 |
| L III D7 | Sparidae | Cheimerius nufar | 780-950 |
170-190 |
| L III D8 | Zanclidae | Zanclus canescens | 790-815 |
190 |
| L III D9 | Sparidae | Rhabdosargus sarba | 790-820 |
170-190 |
| L III D10 | Serranidae | Liopropoma cf latifasciatum | 840-865 |
170-180 |
| L III E1 | Pinguipdidae | Parapercis robinsoni + | 750-865 |
170-190 |
| L III E3 | Sciaenidae | ? Argyrosomus thorpei | 745-790 |
170-220 |
| L III E3A | Sparidae | Acanthopagrus vagus | 720-770 |
190-220 |
| L III E4 | Caproidae | Antigonia rubescens (a cluster of 5 or 6 cryptic spp.) | 815-890 |
180-190 |
| L III E4A | Sciaenidae | ? Johnius dussumieri | 815-890 |
190-230 |
| L III E5 | Macroramphosidae | Macroramphosus scolopax | 865-985 |
200-220 |
| L III E7 | Acanthuridae | Acanthurus mata | 670-720 |
160-180 |
| L III E8 | Chaetodontidae | Heniochus acuminatus | 690-745 |
170 |
| L III E9 | Paralichthyidae | Pseudorhombus elevatus | 730-810 |
50-90 |
| L III E10 | Acanthuridae | Zebrasoma gemmatum | 665-770 |
150-190 |
| L III E11 | Sparidae | Crenidens crenidens | 720-770 |
180 |
| L III F1 | Gadidae | Unknown | 720-840 |
145-170 |
| L III F2 | Chaetodontidae | Chaetodon blackburni, C. dolosus & C. auriga | 700-745 |
170-180 |
| L III F3 | Nomeidae | Cubiceps pauciradiatus | 720-820 |
190-210 |
| L III F5 | Leiognathidae | Secutor ruconius | 650-720 |
170-190 |
| L III F6 | Acanthuridae (Nasinae) | Naso mcdadei, Naso thynnoides & N. brevirostris | 670 |
170 |
| L III F7 | Pinguipdidae | Parapercis maculata & Parapercis schauinslandii | 670-725 |
145 |
| L III F9 | Bothidae | Unknown | 600-695 |
120-145 |
| L III G2 | Pomacanthidae | Centropyge acanthops | 615 |
155 |
| L III G4 | Acanthuridae | Acanthurus triostegus | 625-675 |
140-160 |
| L III G7 | Bothidae | Crossorhombus valderostratus | 550-610 |
120 |
| M I A1 | Veliferidae | Metavelifer multiradiatus | 2210-2300 |
multiple |
| M I A1A | Ostraciidae | Ostracion cubicus | 1945-2115 |
multiple |
| M I A2 | Exocoetidae | Unknown | 1900 |
multiple |
| M I A2A | Soleidae | Unknown | 1800 |
multiple |
| M I A3 | Diodontidae | Diodon holocanthus | 1610-1870 |
multiple |
| M I A4 | Pleuronectiformes | Unknown | 1610 |
multiple |
| M II A1 | Pleuronectiformes | Dagetichthys marginatus | 1335-1440 |
multiple |
| M II A1 | Pleuronectiformes | Unknown (Type B) | 1405-1415 |
multiple |
| M II A1A | Molidae | Ranzania laevis | 1370-1560 |
multiple |
| M II A2 | Scombridae | Sarda orientalis | 1200-1370 |
multiple |
| M II A2A | Soleidae | Aseraggodes heemstrai | 1150-1320 |
multiple |
| M II A3 | Platycephalidae | Unknown | 1100-1225 |
multiple |
| M II A4 | Bramidae | Unknown | 1285 |
multiple |
| M II A5 | Pempheridae | Parapriacanthus ransonneti + other pempherids | 1100-1200 |
multiple |
| M II A6 | Cynoglossidae | Symphurus sp. | 1080-1150 |
multiple |
| M III A1 | Platycephalidae | Rogadius portuguesus | 920-970 |
multiple |
| M III A2 | Platycephalidae | Onigocia oligolepis & ?Thysanophrys celebica | 890-970 |
multiple |
| M III A4 | Creediidae | Apocreedia vanderhorsti | 960-1010 |
multiple (2) |
| M III A5 | Soleidae | Pagusa nasuta (= Solea turbynei,= S. bleekeri) | 750-800 |
multiple |
| M III A6 | Cynoglossidae | Cynoglossus lida | 770-960 |
multiple |
| M III A7 | Creediidae | Limnichthys nitidus | 700-790 |
multiple |
| M III A8 | Cynoglossidae | Unknown | 680-700 |
multiple |
| M III A9 | Unknown | Unknown | 600 |
multiple |
Table 5: The species list (by Order or Family):
Can be seen on the homepage via this link.